Althea Medical
A life-saving development for PE
Highlights
Highlights
A revolutionary technology for the treatment of one of the most challenging cardiovascular diseases-Pulmonary Embolism [PE]
Hundreds of millions of people worldwide are at risk of developing pulmonary embolism, which attacks unpredictably when a blood clot detaches from the deep venous system in the legs and travels to the heart, ultimately blocking the pulmonary arteries. This blockage severely impairs the normal function of the heart, preventing blood flow to the lungs and causing extreme cardiac distress. Nearly half of all patients admitted to hospitals suffering from PE are diagnosed as medium to high-risk patients and require urgent blood clot removal treatment. Conventional treatment of these patients involves a systemic administration of clot-dissolving drugs or open heart surgery. Blood clot dissolution drugs are associated with a high risk of intracranial bleeding, while open heart surgery is extremely invasive and often results in short- and long-term mortality. Althea Medical has developed an efficient treatment for these patients, which allows for quick and safe removal of the blood clot and substantially increases treatment success and long/short-term survival rates. Clinical studies conducted over the last few years clearly show that catheter-based treatments quickly remove blood clots and dramatically increase survival rates, with ~90% of high-risk patients reaching full recovery.
Investment by a leading venture capital fund Peregrine Ventures
Althea Medical received a substantial investment from Peregrine Ventures, a major investment fund focusing on medical device development. The company was founded under “Incentive”, Peregrine’s investment incubator arm, and receives support from Israel’s Innovation Authority (IIA). Peregrine has had multiple acquisitions of its medical device portfolio companies, most notably B-balloon, Valtech Cardio, and Rocketick, which were sold for tens and hundreds of millions of dollars.

Highly experienced team and leading cardiologists on the medical advisory board
Althea Medical was founded and is now managed by a team of engineers with extensive experience in the medical device industry and a long list of successful exits. Rafi Benary, founder and CTO, has 25 years of experience in medical device start-ups. Rafi has already founded seven companies in the field, including Ventor Technologies which was bought by Medtronic for hundreds of millions of dollars. Lia Ofek, founder and CEO, held multiple key positions in medical device companies. Lihu Avitov, founder and board member, is active in the medical device industry for over 20 years and was the CEO of Angioslide when it raised tens of millions of dollars to develop a heart artery-clearing catheter. The company established a medical advisory board made of world-leading cardiologists and experts in pulmonary embolism treatment. Dr. Kenneth Rosenfield, Section Head, Vascular Medicine and Intervention at Massachusetts General Hospital, Boston, serves as the chairman of the company's scientific advisory board. Dr. Rosenfield was also the founder of the international consortium for pulmonary embolism treatment.
.jpg)
Patents nearing approval on innovative catheter technologies
The company has filed patent applications which are in the advanced stages of approval in various countries for two technological breakthroughs that set its technology apart from the competition. Althea Medical’s IP includes tools for quick and safe pulmonary embolism treatment and developments with wider applications that address basic unmet needs in catheter-based treatments. The company is also developing technologies for future implementation to maintain its edge and technological advantage and is currently filing applications that cover these new concepts.

Outstanding results in pre-clinical experiments
Althea Medical has already completed a series of pre-clinical trials that proved its technology efficiently removes blood clots from pulmonary arteries and supported the claims regarding advantages compared to leading devices in the field. The company established early communication with the FDA and received approval for its clinical trial program and a guarantee to apply for the shorter 510(k) FDA track based on a clinical trial with only 75 patients. These prior agreements with the FDA drastically reduce investment risk and enable the company to realistically plan its time and budget towards FDA approval.
Pitch
Pitch
Pulmonary embolism affects roughly 1.5 million people around the world every year. It is induced by many factors including physical trauma, diabetes, heart attack, pregnancy, obesity, old age, surgery, etc. The disease can attack at any age without prior symptoms. A blood clot blocks the pulmonary artery and causes acute cardiac stress and a drastic decrease in blood flow to vital organs. Half of the patients arrive at the hospital in moderate to high-risk conditions and require immediate treatment. The patient’s unstable physical condition means there is a high risk of sudden death during treatment. Existing treatments don’t allow for quick blood clot removal without significantly increasing chances of deterioration and death.
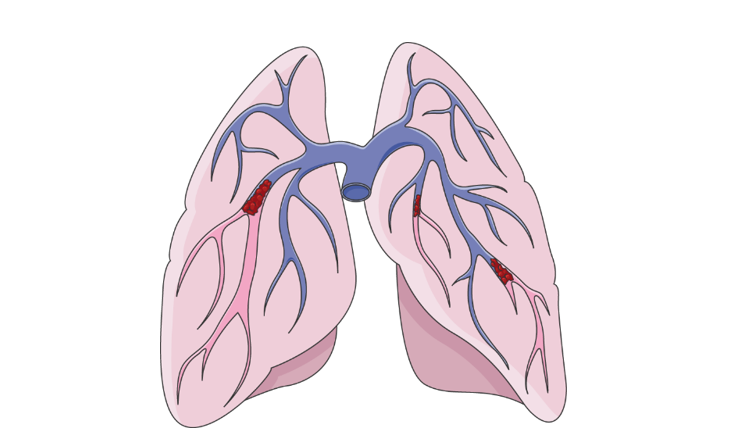
The lung is a unique intersection between air and blood and exhibits a complex set of blood vessels that, like a tree, diverge from thick, large arteries, to thin and delicate capillaries. Blood clots that typically form in the legs, can unexpectedly be released into the bloodstream and occlude these complex structures. Occluded blood flow to this vital organ is life-threatening, and 30% of people diagnosed with pulmonary embolism don’t survive for more than a month. The image below illustrates the blockage of the pulmonary arteries, the closer the blockage is to the main artery, the worse the condition of the patient.
Available treatment options include infusion of blood clot-dissolving drugs or open heart surgery. The use of blood clot-dissolving drugs poses a serious risk of brain hemorrhage and is not recommended for many of patients. Open heart surgery is efficient but extremely invasive, the risk of mortality is high, and recovery times are very long. New technologies that emerged in recent years allow for the treatment of PE using catheterization. The pulmonary embolism field is undergoing a similar process to the one that revolutionized the aortic valve replacement field in the early 2000s. Catheter-based valve replacement methods were introduced as an alternative for patients who could not be operated on, and have since become the standard of care.
Pulmonary artery catheterization has been practiced for over 40 years, primarily used to determine right-heart function. The procedure is used for surgery-free diagnosis and treatment of pulmonary hypertension, heart disease, breathing obstructions, and pulmonary embolism.
There are two catheter-based ways to treat pulmonary embolism:
- Inserting a thin catheter into the pulmonary artery and dripping small amounts of clot-dissolving medication directly into the obstructed area to slowly break the blood clot. The procedure takes 24-48 hours to complete, the patient must remain under close monitoring, and although it is efficient, it involves a serious risk of a brain hemorrhage.
- The second approach involves inserting a large-diameter catheter into the obstructed area and using a vacuum to aspirate the blood clot out of the artery. This procedure typically lasts an hour and is preferred in many cases but requires a very skillful medical team. The procedure is often hampered by technical difficulties caused by the properties of the catheter and its production process. Leading products in the field today have several key disadvantages:
- Limited flexibility makes it hard for the doctor to navigate the catheter through the heart structures and the complicated blood vessels to the obstructed area.
- Structural faults can happen along the catheter, which reduce aspiration efficiency and limit treatment efficacy.
- Some blood clots are too big or are strongly adhered to the blood vessel and cannot be removed with existing devices.
Physicians today have a clear understanding that a minimally invasive treatment tool is needed, that can compensate for all these disadvantages to provide safe and efficient treatment for pulmonary embolism.
The solution
Althea Medical’s engineers approached the problem based on a profound understanding of the disease and related challenges. This understanding of doctor requirements and disease complexity produced a combination of technologies aimed at easy catheter navigation and blood clot removal, and specific solutions for currently untreatable cases.
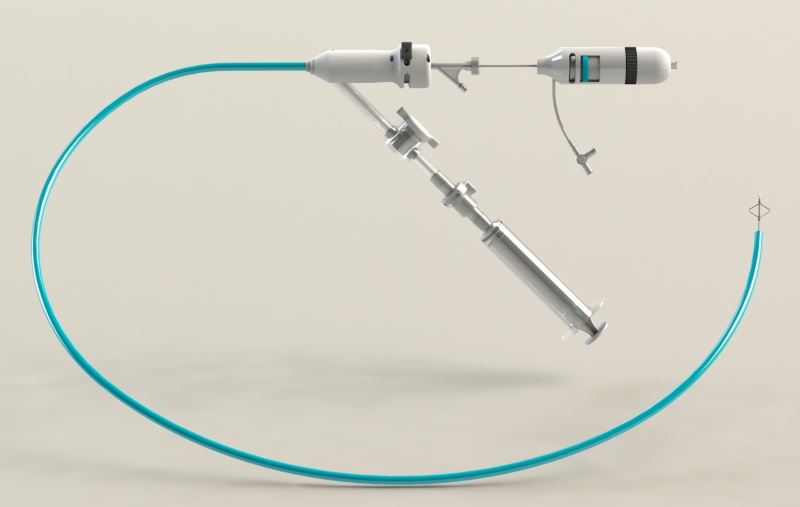
Althea Medical combines two novel technologies into one successful solution. First, new production methods, different from those used until now, enable the company to produce the largest aspiration catheter in the market today while maintaining very high flexibility. This allows for quick and easy access to the obstructed area and more efficient aspiration. The unique production method also increases the structural strength of the catheter which prevents it from kinking during navigation.
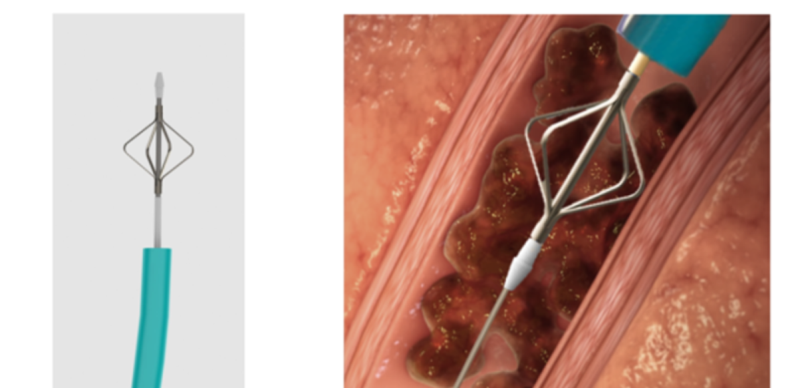
The company also developed a unique mechanical device to treat blood clots that cannot be aspirated. The device is much smaller in diameter and is used to separate blood clots from artery walls or to access blood clots located in arteries that cannot be accessed using the large aspiration tube. Combining these two novel technologies into a single medical device provides physicians with an efficient and safe tool for pulmonary embolism treatment and quick patient stabilization. The device reduces treatment and recovery time and drastically reduces the chances of failure and the need for repeated treatment.
Catheter flexibility:
Center diameter:
.png)
Unique mechanical means for treating clots that cannot be aspirated:
The technology
As in most industries, the medical device field advances with the front of medical engineering. Althea Medical developed a unique process that enables it to manufacture the largest catheter in the market while maintaining the flexibility and structural integrity of the device. These allow for safe and efficient navigation through the arteries and maximal aspiration capacity. Althea Medical’s unique technology takes pulmonary catheterization to a new level and ensures fast, efficient, and safe treatment for one of the world’s most lethal cardiovascular diseases.
Althea in the media:
- The company was chosen to present at the Innovators Day of EuroPCR 2023, the most prestigious cardiology conference in Europe (May 15, 2023). We were honored to be presented by Dr. David Meerkin, a renowned Cardiologist from Shaarei Zedek Hospital with extensive experience in groundbreaking cardiological procedures. Dr. Meerkin is a member of Althea Medical’s Scientific Advisory Board.
- Medtech Strategist’s May 2023 edition features a review of the company in an article about novel pulmonary embolism treatment technologies.
.png)
Team
Team
|
Biography
Lia has a Master‘s degree in Bio-medical Engineering from Tel Aviv University. She made a career transition from management in security-oriented industries to the medical device field, aiming to promote quality medicine and help save human lives. Lia has been focusing on the cardiovascular field ever since, and has held several key positions in various start-up companies, including Endospan, where Lia and Rafi met, and Valtec Cardio. After Valtec Cardio’s acquisition by Edwards Lifesciences, a leading US-based medical devices company, Lia continued her work there until she founded Althea Medical.
|
|
Biography
Rafi is a Mechanical and Materials Engineer with a Master‘s degree from Stony Brook University, New York. He is active in the medical device field for more than 25 years, and already founded and managed seven different medical device companies. Ventor Technologies was purchased in 2009 by Medtronic in one of the most notable exits in the field for hundreds of millions of dollars. Endospan, another company founded by Rafi, is currently in an acquisition process and has a distribution agreement with a major medical device company in the United States. Rafi is active in many aspects of the medical device field, including structural heart, heart failure, vascular disease and Women’s health. Rafi has contributed to the development of Israel’s medical device industry and was involved in establishing a laser cutting factory operating in Israel which acts as a key provider to the industry.
|
|
Biography
Lihu is a Technion graduate in Economy and Industrial Engineering and has held key management positions in investment funds and medical device companies, with more than 20 years of experience in the field. He managed operation overseeing dozens of workers in multiple countries and was the CEO of Angioslide, an FDA and CE approved medical device company. Lihu led Angioslide as it obtained international success, was the CEO of Naiot and a board member for YEDA- a company of the Weissman institute. Lihu also held high ranking positions in Early-Stage Venture Capitals and in XT Venture based in Australia. Today he is also a board member for Revamp Medical, the last in a long list of start-ups he was involved in through recent years.
|
|
Biography
Dr. Rosenfeld is head of the interventional cardiology unit in Massachusetts general hospital in the United States, a world class cardiovascular expert, and head of Althea Medical’s medical advisory board. Dr. Rosenfeld, a world-renowned interventional cardiologist and an expert in catheter-based treatments, if the founder of the international consortium for treatment of pulmonary embolism. Dr. Rosenfield performed thousands of catheter-based procedures during his long career, is involved in all of Althea Medical’s processes, and is a major source of knowledge and support during product development and preparation for the clinical stage.
|





