STStent
Allowing millions to breathe freely
101% of funding target
Highlights
Highlights
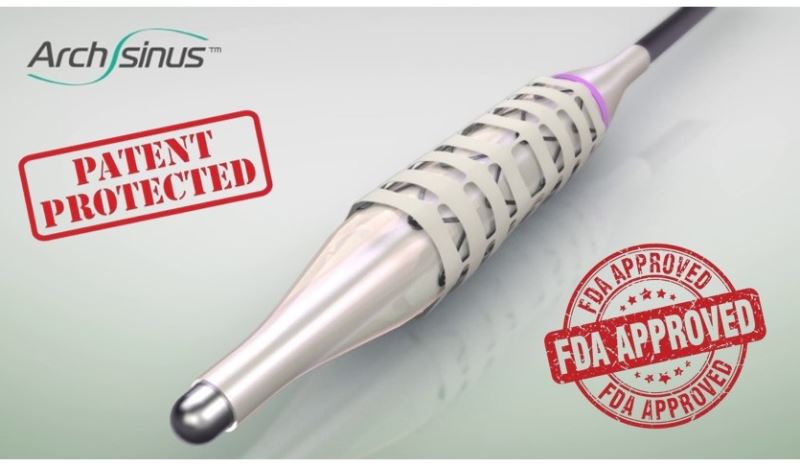
A Patent-Protected Revolutionary Technology that is FDA-Approved for Sale in the USA
S.T. Stent has developed the ArchSinus, a distinctive, temporary stent for chronic sinusitis patients, which is used for sinus implantation following sinus surgery and is expected to completely change the standard treatment for patients. The pioneering device dramatically improves surgery outcomes, prevents inflammation and common complications, and is expected to cause a reduction in the need for revision surgery. The ArchSinus is FDA-approved for marketing and sale and is protected by several strong patents listed in the United States, Europe, Japan, China, Australia, Canada, and Israel.
![]()
![]()
Endorsement and Investments from Leading Innovation Institutes in Israel and Abroad: Trendlines, the Israel Innovation Authority, and Horizon 2020
S.T. Stent operates within the Trendlines Group—Israel’s leading tech incubator, which invested in the current round with Agriline. Trendlines specializes in med-tech investments and is supported by the Israel Innovation Authority. Moreover, the company received the EU’s prestigious Horizon 2020 grant in 2018.

Proven Efficacy in Clinical Trials, Successful Pilots in Leading Medical Centers Around the World, and Commercialization in the United States
The ArchSinus stent has proven its safety and efficacy in two successful clinical trials, which were carried out in top medical centers in the USA and Israel, including Herzliya Medical Center, Northwestern University, and Lenox Hill Hospital. The excellent clinical results have been published in the prominent medical journal the American Journal of Rhinology and Allergy.
The company is currently completing extensive commercial pilots in prominent medical centers in Israel and the United States, such as Northside Hospital, Duke University, Piedmont Hospital, Mount Sinai, Lenox Hill, and more. Several of these medical centers have already purchased the stent and continue to purchase it with repeat orders.

Major Interest in the Company’s Product from the International Medical Community
The company’s technology has sparked major interest from strategic players in the global medical community, following the ArchSinus’ presentation at the annual American Rhinology Society meeting in 2018 and in the RhinoWorld conference in 2019. Some of the field’s most prominent ENT companies, including J&J, Stryker, and Medtronic are closely following the progress of the company. Finally, Baylor Surgical Hospital in Fort Worth, Texas recently because the S.T. Stent’s first paying and repeat customer.

A Team of Internationally Renowned Experts with Broad Med-Tech Development Experience
At the head of S.T. Stent is a strong team with decades of tech, medical, and scientific experience under their belts, in leading companies in Israel and abroad. The team is led by the founder and CEO, Dr. Joseph Flomenblit, who is a medical stent specialist, developer of numerous patent-protected technologies, and a consultant and project manager to conglomerates such as J&J, Boston Scientific, Medinol, and Rephael. Michael Berman, Board Chairman, is an experienced investor and entrepreneur in the medical development field and is on the board of 10 emerging medical companies. Additionally, the company has scientific advisors from prestigious international medical centers, that are currently leading the ENT field in Israel and abroad.

A Broad International Market in Need of an Effective and Economical Solution
There are tens of millions of sinusitis patients in the United States alone, 500,000 of which undergo sinus surgery annually. Half of these patients will experience recurring symptoms and might even need to undergo revision surgery. 90% of this market is left without a solution, due to existing unsatisfactory technologies that fail to prevent the need for revision surgery. The ArchSinus aids in decreasing common complications post-surgery and the need for revision surgery. Thus, the stent is expected to reduce the costs of healthcare services, for insurance companies, and patients alike. Moreover, S.T. Stent’s technology is expected to be beneficial for alternative uses, such as failed nose surgeries, which have yet to be resolved with a proper solution.
Pitch
Pitch
Today, there are 30 million chronic sinusitis patients in the US alone, with similar prevalence in other developed countries around the world. Chronic sinusitis is a severe inflammation of the sinuses, that is caused by an infection or allergy and results in facial pressure and pain, nose congestion, and intense headaches. When drugs fail to provide durable relief, FESS (functional endoscopic sinus surgery) is used to improve aeration and nasal drainage and remove the damaged tissue. Every year, over 500,000 patients in the United States undergo this surgery. However, 50% of these patients experience recurring symptoms, such as inflammation and nasal septum adhesions. 15% of these patients undergo revision surgery.
A true need for a safe and effective solution persists for these patients—one which will enable the sinus cavities to heal in an open and aerated fashion, thus reducing the need for revision surgery and significantly decreasing medical costs.
The Need
Due to unsatisfactory outcomes post-sinus surgery and the common need for revision surgery, different technologies have been introduced to the market in order to meet the existing need, such as different drugs, implants, and surgery equipment. The current stent solution (Intersect ENT, Propel Stent) is expensive, offers poor crush resistance, can be uncomfortable for the user, and can be hard to remove. Furthermore, Propel Stent can CAUSE tissue inflammation after eluting its embedded steroid, thereby exacerbating the clinical presentation.
The Solution
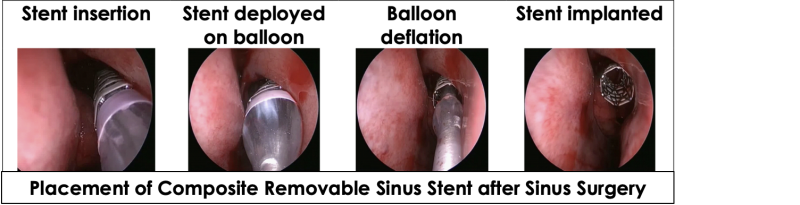
The novel composite ArchSinus, S.T. Stent provides a novel solution for chronic sinusitis patients post FESS surgery. The stent is meant to preserve the corrected nasal geometry post-surgery. It is easy and convenient to insert and remove, maintains patency of the sinus cavity for up to four weeks (sufficient time for tissue healing), aids proper aeration and drainage, enables access to necessary nasal medication, and dramatically improves the post-surgery recovery process. In this manner, S.T. Stent is expected to help prevent revision surgery, reduce treatment costs, and boost healthcare providers’ revenue.
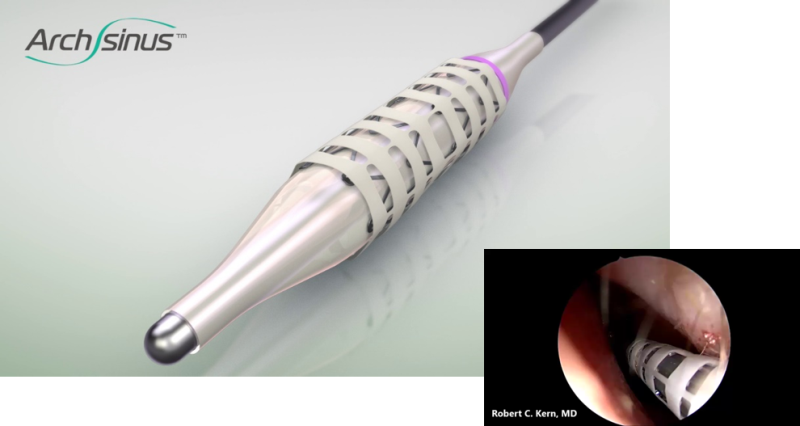
The Technology
The composite ArchSinus developed by S.T. Stent is a nitinol balloon-expandable stent with a biostable polymer outer layer, that allows increased elasticity while maintaining a uniform shape and crush-resistant mechanical strength. These characteristics permit the stent to provide up to 28 days of patency of the sinus cavity. As a result, the sinus tissue can heal better and faster post-surgery. The stent’s implantation process is easy, allows precise positioning, and is perfectly tailored to the unique anatomy of the ethmoid sinus. Once the stent expands, it is meant to support the sinus walls, thus maintaining patency, and preventing middle turbinate lateralization. Moreover, the device aids in proper tissue aeration and drainage and enables the delivery of approved nasal medications via rinse or aerosol. After four weeks, the stent can be easily removed without anesthesia or other invasive procedures.
S.T. Stent in the Media
Team
Team
|
Biography
International nitinol expert; over 25 years expertise in stents; leading intl. projects with J&J, Boston Scientific, WL Gore, Medinol; consultant Medtronic, Boston Scientific, Memry, Rafael Military Systems; Ph.D. in Solid State Physics; above 70 patents
|
|
Biography
Industry Expert, medical device investor/entrepreneur who serves on the boards of 10 emerging medical companies.
|
|
Biography
Broad experience in Sales and Market Development in companies such as Inspire, IntersectENT, EndoGrastric Solutions, Acclarent, Intuitive Surgical. |
|
Biography
Ten years experience in RA and Clinical research. M.Sc. in Biology from Tel Aviv University. |
|
Biography
30 years of development, innovation and problem-solving experience in various fields of technology like medical devices, microelectronic components, precise mechanics and robotics etc.; M.Sc. in Engineering. |






