SynCath
Revolution in the field of neurosurgery
16% of funding target


Highlights
Highlights
Breakthrough technology for data-based treatment of brain injuries
SynCath's innovative solution utilizes advanced cardiac-synchronized balloon-catheter, providing neurosurgeons with critical data regarding patients’ medical condition in cases of severe head injury or cerebral hemorrhage and allowing them a novel, highly effective physiology-based, minimally invasive therapy. The system's efficacy and safety have been proven in extensive preclinical trials, and it is meant for use as a tool to increase blood flow to the brain. Furthermore, by preventing an intra-cranial-pressure crisis, it is designed to prevent complex and dangerous surgeries, when unnecessary, and alert their necessity, in the few unavoidable cases.
Exclusive IP developed by expert clinicians and scientists from Tel Aviv University, Hadassah Medical Center and NYC Lenox Hill
The tech was developed by Prof. Ofer Barnea and Dr. Omer Doron, in collaboration with Prof. Guy Rosenthal, Director of Neurosurgical Intensive Care at Hadassah, by combining elements of personalized medicine, machine learning, interventional neurology and advanced mathematical modeling. These experts have succeeded in creating a groundbreaking technology for neurocritical treatment, for which SynCath holds the exclusive WW license. The patents used for the technology are in advanced registration stages around the world (already granted in China) and constitute an attractive and exclusive intellectual property.
Supported by the Israel Innovation Authority and great interest from leading entities in Israel and worldwide
SynCath has received a grant from the Israel Innovation Authority as part of the institutional KAMIN program, as well as support under the start-up companies program. The company's tech is also raising much interest among international institutions and experts. SynCath has already been in contact for collaborations with leading multinational companies in the field. A long line of medical experts have already expressed interest in using the company's technology, from leading institutions including NYU Langone Health Center for Stroke and Neurovascular Diseases, NYU, Lenox Hill Hospital, The Center for Neurorestoration, USC, Northwell Health, Baylor College of Medicine, Chicago Medical Center North Shore University Hospital, LIJ Medical Center, Charite in Berlin and more.

Addressing a rapidly growing market, estimated at billions of dollars annually
The company's tech is designed for the use of the neurosurgical intensive care market, as well as the fast-growing trauma and stroke treatment markets, with hundreds of thousands of cases in the US, and over a million cases in Europe each year, requiring the kind of treatment for which SynCath's development is intended. The company expects that its technology could, from the get-go, be used to treat over 1.5 million patients every year, with a relevant primary market potential of billions of dollars. SynCath offers this huge market the first medical device of its kind in the world, capable of saving lives and becoming the next standard for treatment in the field.
Leading team of experts with vast experience in medical devices and business management
SynCath is led by a team of experts in the fields of engineering, medicine and business management. The company's team members have decades of experience in developing diverse medical equipment, as well as planning, conducting and publishing numerous clinical and preclinical studies, developing intellectual property and registering patents worldwide, managing and developing business, raising resources, handling regulatory approval (FDA, CE) and more. The company also enjoys the support of leading medical advisors from world-renowned institutions, leading the company's clinical R&D, including well known specialists in the field of neurological intensive care - Prof. David Langer, Director of Neurosurgery at Lenox Hill Hospital in New York, Prof. Guy Rosenthal, Director of Treatment The neurosurgeon at Hadassah, Prof. Erez Nosek of NYU Langone, and Prof. Nino Stocchetti from Milan.

Pitch
Pitch
The Need
Neurosurgical injuries in general, and traumatic head injuries particularly, usually happen surprisingly, at almost any age, as a result of a variety of events: from sports injuries, car or bicycle accidents, to stroke or violent events – all of which require urgent and effective medical care to lessen the possibility of irreversible damage to the brain and prevent loss of life. The brain damage caused by traumatic brain injury is irreversible and may damage the quality of life, creating a dependence on caregivers, at a heavy financial cost. Being given the right treatment, at the right moment, may prevent the loss of additional brain cells.
In traumatic head injury (TBI), subarachnoid hemorrhage (SAH) and bleeding due to hemorrhagic stroke and aneurysm (ICH), the result is a significant increase in intracranial pressure, as a result of edema in brain tissue. The increase in pressure causes blood vessels to partially collapse, leading to a significant decrease in blood flow to the brain, and the supply of oxygen to the brain tissue, accordingly.
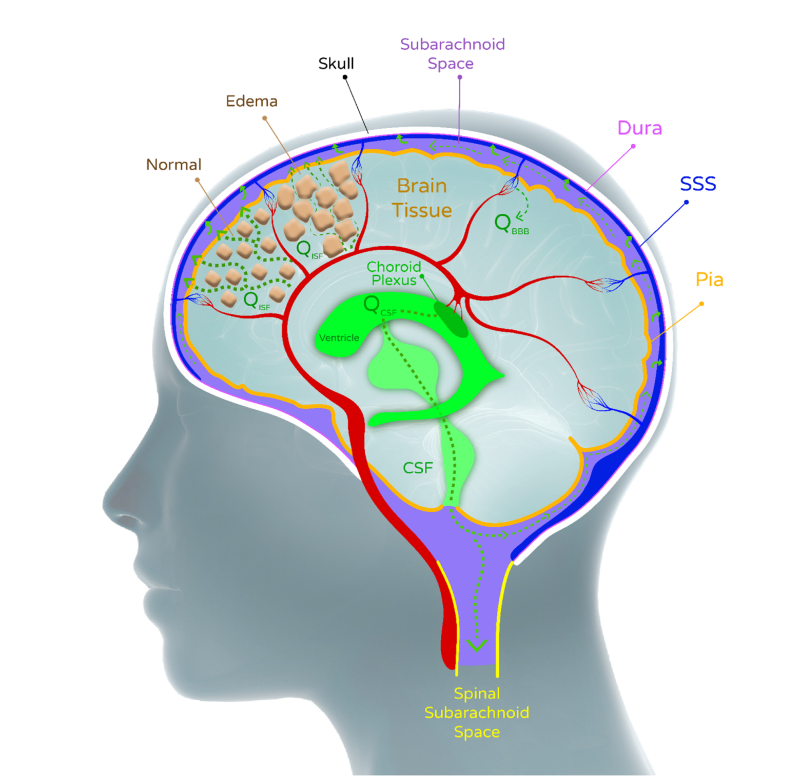
The immediate form of treatment available today is limited in terms of how much and for how long it can actually help these patients. Current treatment includes Mannitol solution (polysaccharide) to extract fluids from the brain cells and so relieve some of the intracranial pressure, inserting intracerebral drainage into the brain for expelling fluids, and finally the removal of a large portion of the skull to allow brain tissue to expand and lower this pressure. Mannitol has a limited effect since along with the CSF fluid removal, other liquids are also drained from other body tissues, resulting in a decrease in general blood pressure, which leads to a decrease in the blood supply to the brain.
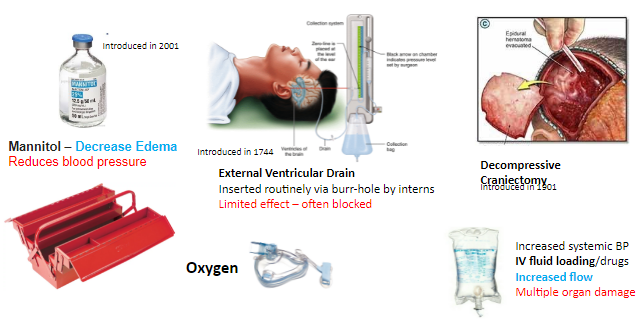
The catheter used currently may be useful, but it often gets blocked by blood clots or the collapse of the ventricles. Catheter insertion is a simple procedure performed by interns. But the removal of part of the skull, on the other hand, is a highly invasive and traumatic process, which is not always effective since the brain tissue has a limited ability to expand. The patient often remains this way for many months, until recovery and skull restoration. A possible indirect treatment is to raise systemic blood pressure to increase flow - but this method may cause injury to other organs such as the kidneys.
These treatments, use of catheter and the partial removal of the skull, are medical procedures which were invented more than a hundred years ago. The methods of treatment are still currently limited and so there is a great importance and a significant need for the development of new tools for treatment, in cases where significant impairment of brain functions or even death may occur.
The Solution
Monitoring intracranial pressure is a critical procedure in treating brain injuries, but the existing process does not allow the neurosurgeon to understand what is really going on inside the skull of the patient, as it's based only on general statistical data rather than on the individual patient. The problem is that for each patient the level of critical pressure, at which dramatic intervention is needed, is different. This is where SynCath's innovative Tulip™ device comes into play, providing neurosurgeons with an innovative and effective tool for assessing the exact condition of each and every patient.
The critical parameter which the device measures, which is not available to neurosurgeons yet, is the intracranial elasticity, and it estimates the amount of fluid that can still accumulate in the brain before high pressure breaks the brain tissue (herniation) and causes severe damage. Another result of the intracranial pressure is also a partial collapse of blood vessels - SynCath's tech addresses this issue as well, as it includes the ability to also assess the blood flow in the brain.
The same device, when operated in a different mode, acts as an ingenious pressure regulator, augmenting blood flow in the brain and increasing oxygen supply to brain cells, thereby reducing the death of brain cells.
How does it work?
A catheter with a balloon at its tip, integrated with the commonly used drain, is inserted into a brain cavity in the same manner as drains are inserted. It is momentarily inflated by 1-2 ml at a specific time in the cardiac cycle. For the monitoring application, this perturbation is performed once every ten minutes. When operated as a pressure regulator, it is inflated momentarily at each cardiac cycle.
The two monitored parameters are estimated based on active monitoring, as opposed to conventional passive monitoring using a sensor - such as in a Manometer. In active monitoring, on the other hand, a deliberate and specific perturbation is caused and the patient's response to it is measured - which allows for the intracranial elasticity and blood flow to the brain parameters to be calculated.
In the treatment mode of operation the balloon is inflated in optimal timing, relative to the cardiac cycle, once in each cycle. The action of rapid filling and emptying at the right timing produces the action of a pressure regulator, which increases the blood flow in the brain and also the supply of oxygen to brain cells, to keep them alive. The company intends to expand this technology through further development to allow the use of a balloon to modulate the intracranial pressure, in a way that will increase the outflow of fluid through the draining catheter.
The Tulip™ device, the revolutionary solution offered by SynCath, will include the current treatment option, in the form of intracranial catheter, with the critical addition of the pressure measurement sensor - providing doctors with a full toolset to finally allow access to all currently available monitoring and treatment capabilities, topped off with SynCath's innovative monitoring and treatment capabilities.
Following discussions and consultations the company has held so far with well-known opinion leaders in the neurosurgical field, experts from around the world have stated their interest in using the company's tech in treating their patients.
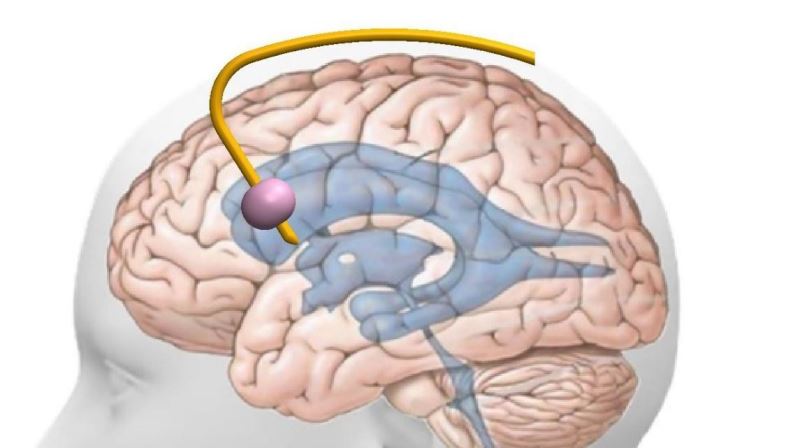
The company's tech is divided into advanced treatment and diagnostic products:
Diagnostic (first commercial stage)
- Intracranial elasticity monitoring to assess the severity of edema and predict upcoming pressure crisis (proven in pre-clinical trials and published in the leading scientific journal in this field)
- Brain blood flow monitoring (in advanced development)
Therapeutic (second commercial phase)
- Inflatable-balloon pressure regulator for increasing the blood flow and oxidation of tissues in the brain (proven in pre-clinical trials, published in the leading scientific journal in the field)
- Controlled active catheter (in development)
Treatment of ischemic stroke
- Insertion of a catheter through a large vein and operating it as a pressure regulator to increase collateral blood flow in an obstructive stroke (future development)
Team
Team
|
Biography
BSc in Electrical and Electronics Engineering and MSc in Biomedical Engineering from Tel Aviv University, PhD in Biomedical Engineering from Drexel University (USA). Specialist in biological flow systems and analysis of the interaction of these systems with medical devices, such as optimization of failing heart care systems and optimization of patient care and management modes. Extensive experience in the design and construction of medical devices. Was a partner in the establishment of industrial ventures in the field of medical devices and systems development, as a consultant for companies in the field and as entrepreneur. He started this project at Tel Aviv University as part of the Innovation Authority‘s support under the KAMIN program. |
|
Biography
BA in economics and a master‘s degree in business administration from the Hebrew University. Was an active director and or part of the founding team / entrepreneur, in several different companies such as Stratasist (public company in the field of 3D printing) - CFO, Idanit Technologies (today HP LARGE FORMAT) - CFO, Gemtex (a printing startup) - Business Development Manager and CFO, Shmerling Engineering (a company that manufactures and sells power plants) - Active Director, Polishek (a public company that produces shade nets for agriculture) - Director, Symbionics (American start-up company in the medical field) - VP of Finance , Image - Entrepreneur / Director, etc. In addition, served as a senior consultant of businesses such as the Scitex Group, Mig Group, Infiniti Group, VC Dekura Group, Alan Yaglom Group, etc. |
|
Biography
An airborne military doctor, specializing in neurosurgery at Hadassah Medical Center. He is currently completing his doctorate in biomedical engineering at Tel Aviv University. Dr. Doron has extensive practical experience in clinics as well as a great interest in the mechanics of brain flow. As part of his doctorate, he developed mathematical models to explain the interaction between many complex factors within the skull, and has paved the way for a new understanding of the relative contribution of each factor, pointing out the possibilities for dealing with complicated cases. He is one of the inventors of the Tulip™ product, and is currently a senior neurosurgeon in advanced training in endovascular surgery, in New York and Boston. |
|
Biography
BA in mechanical engineering from Tel Aviv University. Was a project manager at a medical products company for the treatment of aortic aneurysm. Involved in all stages of development and testing, including preclinical trials and production. Experienced in the design and characterization of systems for V&V testing. Development and production of machines for production and quality control purposes. His experience focuses on developing a catheter from initial characterization to laboratory and pre-clinical / clinical trials. |
|
Biography
Has completed his studies in electrical engineering at Tel Aviv University (master‘s degree with honors) with an emphasis on mathematics, physics, and software. 18 years of experience in establishing and managing medical device companies, raising resources (millions in total), developing business models, management at the basic / applied research stages, development, definitive clinical trials, and initial market penetration stages. Extensive experience in the fields of R&D and engineering. Invented approx. 80 patent families - mostly in the fields of medical technology. Co-author of groundbreaking publications in professional medical journals. Experienced in appearing and speaking in front of an audience - investors as well as scientists, technology, and medicine crowds. |
|
Biography
With a doctorate in medicine, Dr. Hornik has shifted his professional focus to the development of medical devices. Served as medical director of Rimed, superDimension, Cardiosonix and ElMinda. He has advised dozens of companies on regulatory processes and FDA submission, pre-clinical trials and clinical trials, as well as development of a regulatory strategy. He was the inventor and entrepreneur at Neetour for non-invasive measurement of CO2 in exhaled air. Dr. Hornik has vast experience in the regulatory field with a very broad vision on the medical and engineering side, and his contribution to SynCath is critical regarding its strategic planning and execution. |
|
Biography
Director of the Department of Neurocritical Therapy, Hadassah Medical Center
|
|
Biography
Director of the Department of Neurosurgery and Vascular Medicine at NYU, New York
|
|
Biography
Director of Neurosurgical Intensive Care at Ospedale Maggiore Policlinico, University of Milan
|
|
Biography
Director of the Department of Neurosurgery at Lenox Hill Medical Center, New York
|









