Escala Medical
364% of funding target


Highlights
Highlights
Helping millions of women worldwide
As many as half of all women are affected by Pelvic Organ Prolpase; 30%-50% of women face POP during their lifetime. Moreover, the prevalence is expected to double in the next years due to the aging population.
Escala’s repair system will change the way POP is treated today by shifting the point of care from the hospital to the doctor’s office, making it accessible to millions of women worldwide.
This shift aligns with the company’s goal to transform POP repair approach to dramatically improve outcomes and patient experience.
A breakthrough repair alternative for an untapped patient population
Currently, surgery is the only repair solution available for POP; yet, it is offered to fewer than 15% of patients. Today, women either compromise on their quality of life or wait until their condition deteriorates to a stage that requires surgery.
The incision-free, non-surgical solution by Escala, allows POP repair in an office setting.
The breakthrough procedure can be performed in just 20 minutes. It provides a new treatment alternative that considerably reduces patient discomfort, recovery time and associated costs.
Invented by experts and backed by industry leaders
Escala’s team combines proven leadership, innovation and experience in women’s healthcare. Founded in 2014, the company is led Dr. Edit Goldberg, an experienced executive in the medical device field with a diverse background in all stages of product development, from conception to commercialization. The medical advisors and inventors of the technology, Prof. Douglas Scherr and Dr. Roger Goldberg, are surgeons in leading US medical centers and are regarded as key opinion leaders in urology and urogynecology. Together, they have a history of successful developments and exits in this market.
The board of directors includes senior management of The Trendlines Group, a leading early-stage investor in Israel that brings decades of entrepreneurial experience to Escala.
Regarded as a promising company, Escala received grants from the Israel Innovation Authority.
Women’s health is a growing multi-billion dollar market
More than 3,300,000 women are diagnosed with POP, with associated costs exceeding billions annually in the United States alone. Current treatment alternatives are limited to supportive devices and surgery. The prevalence of POP is expected to double in the next years.
The market is dominated by large international medical device companies creating a competitive M&A landscape. Escala’s solution has already produced strong strategic interest as the total addressable market for the device is estimated in the billions of dollars. Escala is geared to success, looking to introduce the device into market in 2020.
Pitch
Pitch
The Need
Currently, POP is repaired only with surgery. A non-surgical, incision-free alternative is not available
Pelvic Organ Prolapse (POP) is a painful, debilitating medical condition with a profound impact on the quality of life of many women. It occurs when the normal support of the vagina is lost, resulting in the “sagging” or dropping of the female pelvic organs. POP is a very common yet quite painful and bothersome condition affecting millions of women all over the world.
Though POP and its associated disorders are rarely life threatening, they have a direct and profound impact on quality of life (QOL).
Current treatment alternatives for women with POP are insufficient and limited. Supportive devices (pessaries) do not repair, and provide only a temporary solution. Repair surgeries are invasive and may be associated with short and long-term complications. Currently, there is no solution for women that had failed the supportive treatment, that are reluctant to undergo surgery or that poor surgical candidates. These women are forced to compromise on their quality of life as a non-surgical, repair treatment alternative is currently not available.
Escala adresses that need with the first non-surgical, incision-free, repair device for Pelvic Organ Prolpase.


.png)
.png)
.png)
.png)
The Idea
With Escala’s device POP can now be repaired in the doctor’s office
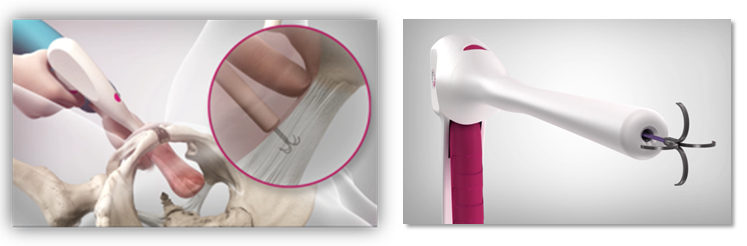
The Escala device addresses a long, over-due need, offering a prolapse repair solution that can be performed as an office-based procedure rather than one requiring surgery. The Escala incision-free procedure does not require longer than 20 minutes to perform. As no cuts are involved the procedure may be performed at the doctor’s office.
The Escala repair procedure is the first and only incision-free repair solution for prolapse. It restores anatomical positions and organ finctionality in three simple steps.
The Technology
Escala’s 20 min. repair procedure represents a long over-due treatment for POP
The Escala repair device is a truly minimally-invasive system that is safer and easier to use compared to current solutions. The procedure is conducted with no incisions. The device combines an anchor, sutures, and a securing element delivered by a designated applicator. Unlike any other device, the Escala device may also be retrieved and removed during the procedure, further enhancing its safety.
The Escala system comprises of well-defined materials, widely used in the medical device industry. The production methods and processes are also common and no special processes are implemented in the manufacturing of the device.
The device was extensively tested in animal trials and cadaver studies with 100% success and is protected by pending global and US patents.
The Escala procedure is easy and quick to perform, and simplifies repair procedures requiring the highest degree of surgical skill. It eliminates the need for deep dissection and simplifies the repair procedure, thus minimizing associated short and long term complications.
IP
Protected technology
Escala has two patent families. The patents, in national (USA, EU, China and Brazil) and PCT stages, strongly protect the device and protecting the technology as well as preventing others from performing an incision-free prolapse repair.
The anchoring unit is the most significant component of the device. Successful prolapse repair is an outcome of sufficient resistance to pull out, as well as an accurate, trauma-free and safe delivery. The Escala umbrella-shaped anchor is the only one meeting all the clinical safety and performance requirements for the following reasons:
Together with the designated applicator providing controlled and accurate delivery and retrieval, our device is the only system designed for a complete incision-free, optimal prolapse repair.
The company’s IP was extensively evaluated by a leading IP firm as part of the due diligence conducted by Trendlines Medical prior to company establishment. In addition, the IP was evaluated by a larger international medical device company following which a term sheet for strategic investment was signed.
How does it work?
Repair is achieved in a safe, three simple steps procedure usually taking no longer than 20 minutes to perform. Unlike other repair solutions, as no incisions are involved, the Escala device may be used both at the hospital and the doctor’s office.
.png)
The Escala 20 min. repair procedure:
1. Position
The surgeon identifies a known landmark in the vagina by palpation and directs the tip of the applicator alongside his finger until it rests against the vaginal wall.
2. Deploy
Squeezing the applicator’s trigger delivers the anchor through a needle in a very controlled manner and to a limited depth. Retracting the applicator leaves two sutures in the vagina.
3. Secure
a button-like securing element is threaded over the sutures and advanced all the way to the vaginal wall. The sutures are then tied and cut. The procedure may be repeated twice on each side.
Team
Team
|
Biography
Over 15 years of medical device industry experience in senior development, strategic and business development positions. |
|
Biography
Senior corporate manager, vast experience in developing & marketing medical devices and pharmaceutical packaging. Technion graduate.
|
|
Biography
Clinical Associate Professor. Director, Division of Urogynecology. The University of Chicago Pritzker School of Medicine.
|
|
Biography
Professor of Urology. Clinical Director, Urologic Oncology, Weill Medical College of Cornell University.
|
|
Biography
Founder of several medical device start-ups, broad management experience in life sciences and medical device companies.
|
|
Biography
Chairman and CEO, The Trendlines Group and Trendlines Medical.
|
|
Biography
CEO Trendlines Incubators, Vast experience in leading life science companies.
|