Omeq Medical
OMEQ has developed a breakthrough product in the field of epidural injections, used to relieve labor pain,
570% of funding target
Highlights
Highlights
Safety & Confidence in Epidural Injections
Today over 40 million epidural injections are performed each year! The injection of anesthetics or pain relievers into the epidural space is very common in child birth, pain management and surgery. During the procedure, the doctor inserts a needle into the epidural, a small anatomical area surrounding the spine. Today, the subjective injection method relies mainly on the doctor's dexterity, skill and experience and in many cases (about 30%), the first attempt is unsuccessful. In 5% of cases inadvertent penetration into the wrong area causes complications. These complications can lead to severe headaches and nerve damage, which prompt additional complications and extended hospitalization, great discomfort plus additional expenses.

The proven technology of OMEQ, significantly increases the accuracy of epidural injections on the first attempt! The novel technology is protected by several international patents (granted and pending) and is compatible with existing practices, allowing for a fast Time To Market.
A Revolutionary Product Proven Effective in POC Research
OMEQ's innovative product significantly increases the accuracy, safety and quality of the injection procedure for women in labor, patients undergoing anesthesia and back pain treatments. Product efficacy was demonstrated in a pre-clinical Proof of Concept (POC) study conducted & supervised at Assaf Harofeh Hospital. In these trials, the company showed over 90% success in identifying the epidural space on the first try. Within a few months, the company will begin its first approved human clinical trial.
Investment by Leading Medical Incubator in Israel
OMEQ Medical is an esteemed company that has received significant investment from The Israel Innovation Authority (IIA, formally Chief Scientist) & TRENDLINES, the leading biotech incubator in Israel. Both groups have performed an extensive Due Diligence and have been joined by private investors.

A Winning Team of Medical and Tech Experts
OMEQ was founded by two young enthusiastic entrepreneurs educated & experienced in medical devices and personally affected by missed place epidural injections. The team consists of medical specialists in anesthesia and pain management, alongside experienced engineers, regulatory consultants and business development Personnel. The company has also appointed an advisory board of internationally renowned experts from Israel (Rambam Hospital), Europe (Bologna University Hospital) and the US (Johns Hopkins Hospital).
Pitch
Pitch
The Challenge
Epidural injections are most commonly associated with labor and child birth. Additional procedures may include regional anesthesia and pain management. Without the aid of fluoroscope (X-Ray) equipment which is banned from delivery rooms and not always available or recommended in other treatments, doctors have no positive assurance of reaching the epidural space with the needle tip. OMEQ's novel Epifinder notifies the doctor and successfully resolves this problem.
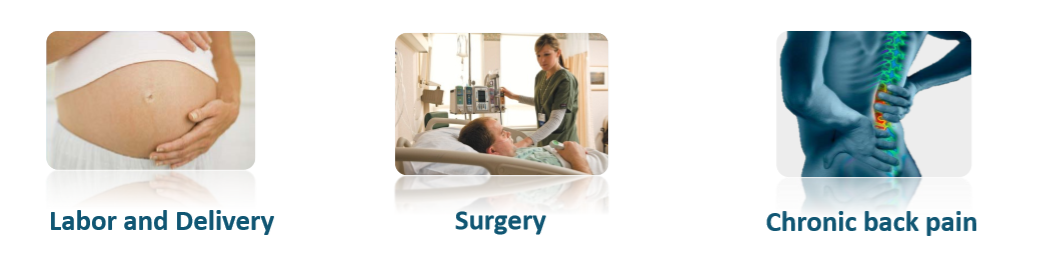
What is an Epidural Injection?
The injection is named after the epidural space, an anatomical area of the body that surrounds and protects the spine. This very small space to which the needle is inserted is only 2 to 4 millimeters wide. In order to perform the injection, the doctor gently and carefully inserts a needle attached to a syringe through the vertebrae (usually in the lower back) until he feels a change in the resistance and concludes that he has entered the right space. Once the doctor believes that the needle is in the proper location he injects the medication to perform regional anesthesia by blocking the pain signals in the spine.
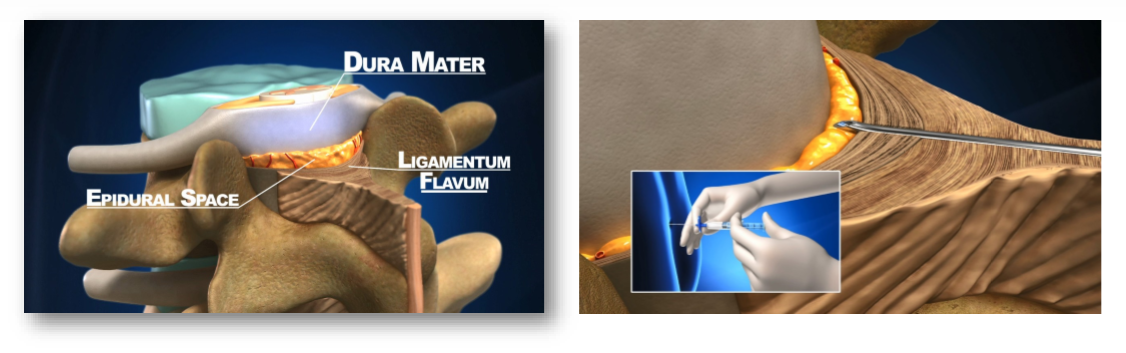
The current technique for identifying the epidural space used by the doctor is a "blind technique" based on the sense of touch, a technique that has not changed in the last 100 years. Identifying the space is difficult, requiring skill and experience. Studies have shown that 30% of the blind technique injections are inserted initially into the wrong place.
Implications of Injection in the Wrong Place:
- No anesthesia or blockage of pain - failure of treatment
- Further attempts will be made to perform a successful injection which significantly extends the duration & cost of the procedure
- In 5% of cases, accidental spinal cord injury will occur, with the chance of complications, including: severe headaches, nerve damage, paralysis, and even death. These complications also prolong hospitalization time and cost.
The Solution
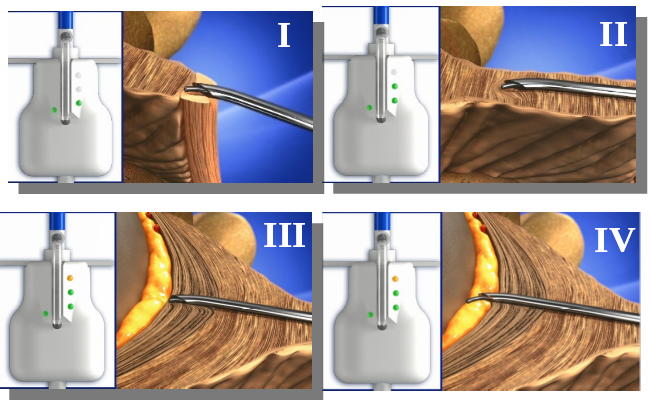
OMEQ has developed a disposable medical device that is fitted between the standard needle and a standard syringe. The device includes a unique electro-mechanical mechanism that measures the resistance exerted by the tissue at the tip of the needle. By monitoring the change in resistance the Epifinder identifies the epidural space and notifies the doctor. The device is intuitive and allows the doctor to perform the injection without changing his technique.
In the pre-clinical trials conducted by an accomplished anesthesiologist the success in identifying the epidural space was 90%.
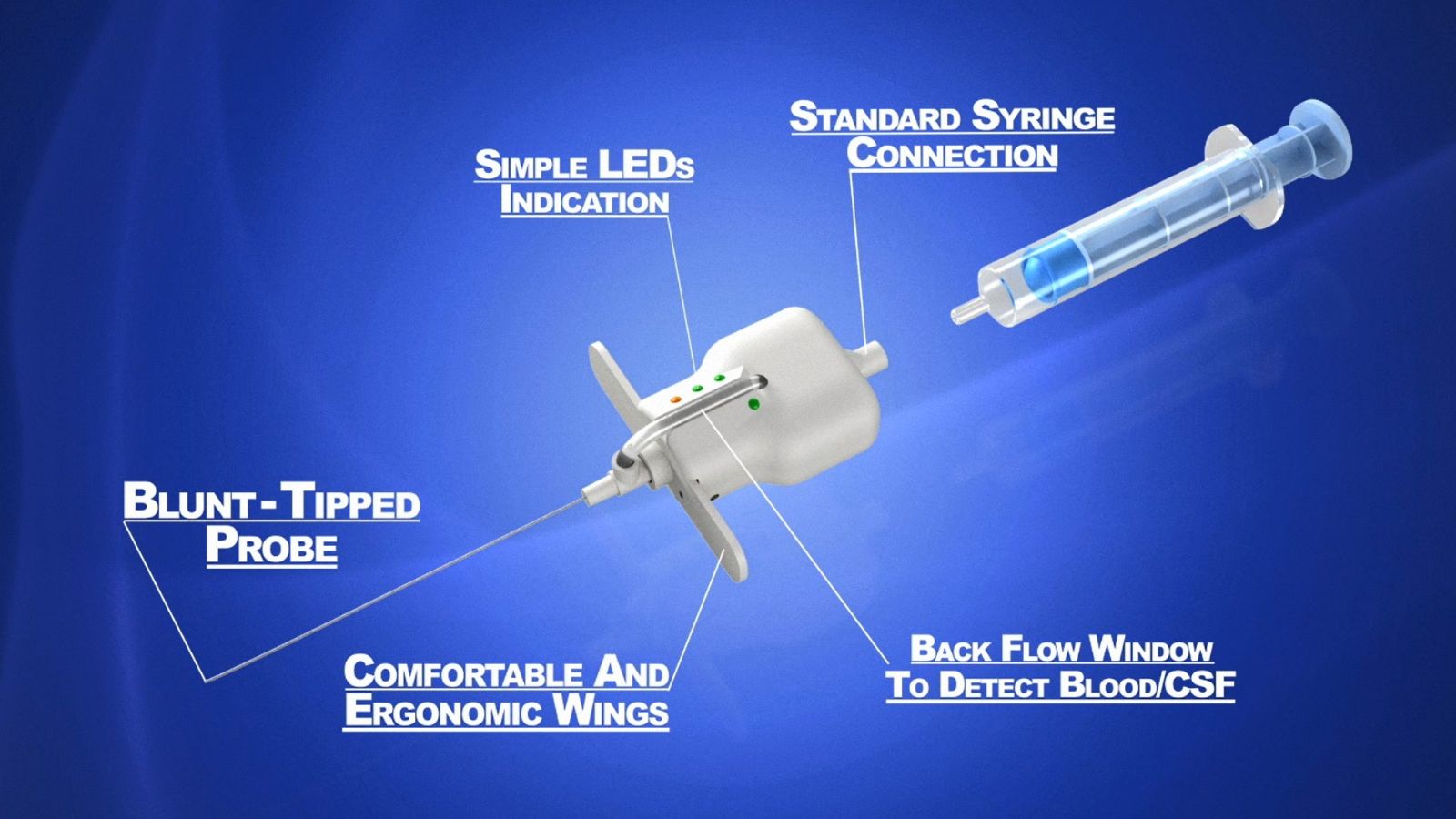
How Does it Work?
The Epifinder includes a thin probe that is connected to a sensor that is wired to an integrated circuit. The probe passes through the needle and repeatedly exits the tip of the needle thus measuring the tissue resistance. An embedded software analyses the measurements, identifies the epidural space and notifies the doctor via LED lights.
The company has 3 international patent applications that protect the sensing technique and the device design. Two patents have been issued in China and the other fillings are in different stages with the local offices.
Our Epifinder
• Significantly increases patient Safety and Quality
• Eliminates additional costs as it reduces the probability of complications
• Simple and easy for doctors to adopt
• Expected annual savings to the medical system of more than half a billion dollars a year
• Disposable
• Cost effective
Team
Team
|
Biography
A medical device professional with more than 20 years of experience in senior management positions at early-stage medical device companies. Specializes in bringing innovative medical devices from concept to commercialization. Assaf has broad experience in establishing and leading multidisciplinary teams. Prior to joining Omeq Medical, worked for early-stage medical device companies and technological incubators, e.g. Sanoculis Ltd., CorAlert Ltd., Aidoc Ltd., Orpheus Medical Ltd., Bio-Gaming Ltd., Regentis Ltd., EyeControl Ltd., Mor-Research Applications Ltd., Tel-Aviv University, ClearCut Medical Ltd., and BrianQ Technologies Ltd. Assaf holds a B.Sc. in Software Engineering and Industrial Management from the Tel-Aviv Engineering Colleague, M.Sc. in Bio-Medical Engineering and M.A. in Philosophy both from Tel-Aviv University
|
|
Biography
Prof. Elon Eisenberg, M.D., is Director of the multidisciplinary Pain Relief Unit at Rambam. He is also a senior lecturer at the Faculty of Medicine of the Technion - Israel Institute of Technology. His main areas of research include mechanisms and treatment of neuropathic pain including CRPS, cancer pain and opioids. Prof. Eisenberg has published over one-hundred and twenty articles, book chapters and abstracts on various areas of pain. Prof. Elon Eisenberg graduated from Sackler School of Medicine, Tel-Aviv University, completed a residency in Neurology, at Rambam Medical Center, Haifa Israel, and a Neurology-Pain Fellowship at Massachusetts General Hospital, Harvard Medical School in Boston.
|
|
Biography
David Gichtin, M.D., is currently a Clinical Associate in the Department of Anesthesia and Critical Care Medicine at the Johns Hopkins Hospital in Baltimore Maryland. He holds a volunteer teaching position in the Pain Clinic at the Ichilov Hospital in Tel Aviv. He is Board Certified in Anesthesia and Pain Management and received his Graduate training at the Johns Hopkins Hospital. He has more than 20 years of experience in the Pain Management field. During this time he held the Chairmanship of the Pain Division at Franklin Square Hospital and was the owner of a successful pain private pain practice in Baltimore Maryland.
|
|
Biography
Boaz Samolsky, M.D., is an Aggregate Professor and Investigator of Anesthesia, Intensive care and Pain Medicine at the department of Medical and Surgical Sciences of the University of Bologna, Italy. Dr. Samolsky serves as a senior Anesthesia, Intensive care and Pain Medicine Consultant at the University of Bologna Teaching Hospital Policlinic S. Orsola Malpighi. He is responsible of the Hospital’s inpatients Acute Pain Service and the Center of Pain Therapy for outpatients with chronic pain. From 2015, Dr. Samolsky serves as a Scientific Director of the International Foundation "NO Pain Foundation". Dr. Samolsky graduated ‘cum laude’ from the Bologna’s University School of Medicine, Italy.
|
|
Biography
Seth Kaufman, M.D., is an Assistant Professor of Addiction Medicine at University of TN Health Science Center in Memphis, TN. He is board-certified in Anesthesiology, Addiction Medicine, Pain Medicine as well as Hospice and Palliative Care. Dr. Kaufman is a graduate of McGill University Medical School and completed his fellowship training at Columbia Presbyterian Medical Center in New York.
|
|
Biography
Omri is a project manager in Inomec, an R&D services company specializing in the development of medical devices. Prior to Inomec, Omri served as an R&D team leader in Omeq Medical and was in charge for the development and V&V phase of the product, and is currently supporting development work conducted for Omeq as part of his role in Inomec. Omri specializes in the field of therapeutic ultrasound and cardiovascular devices. Omri holds a B.Sc. in Bio-Medical engineering from the Afeka college of engineering.
|
|
Biography
Chairman and CEO, The Trendlines Group Chairman, Trendlines Medical
Todd Dollinger brings decades of entrepreneurial experience to Trendlines. He serves as the Chairman of Trendlines Medical Singapore and as Investment Committee chair of Trendlines Incubators Israel. As well, Todd is a director and chairman of a number of Trendlines’ portfolio companies. He founded The Trendlines Group with Steve Rhodes in 2007 and shares the positions of Chairman and CEO of Trendlines with Steve. Together, they lead the Group’s strategy. |
|
Biography
Investor and member of the BOD, Jonathan (Yoni) has vast experience in developing & marketing medical devices and pharmaceutical packaging. Jonathan hold a BSc. and MSc. in Mechanical Engineering from the Technion Institute of Technology and an MBA from Bradford University in the UK. He contributed his expertise and experience to the company‘s management
|