Aqueduct Medical
178% of funding target
Highlights
Highlights
Successful Clinical Trials in the US and Europe
The company has completed extensive and successful clinical trials in five centers in Europe and the United States, demonstrating the effectiveness of the cervical dilatation device and its significant advantages over existing devices. The trials were conducted with the first device developed by Aqueduct Medical – the AQUEDUCT-100; now the company is preparing for trials with the second device – the AQUEDUCT-200, which is in its final stages of development.
Fully Owned Intellectual Property and Regulatory Approvals (CE, FDA)
Following the success of its clinical trials, the company received CE and FDA approvals for AQUEDUCT-100, and is currently on the FDA Special 510k fast track for approval of AQUEDUCT-200, which it expects to receive in July 2020. At the same time, the company will also be working to obtain CE approval.
In addition, the company has a US patent for the AQUEDUCT-100, and a second patent for the AQUEDUCT-200 has been filed in Israel, the United States, Europe, China and Japan, and are currently in the National phase (Patent Pending).

Strategic Collaborations and International Commercial Activity
Aqueduct is currently preparing for a joint clinical trial in Tokyo with MCMed - the Mitsubishi subsidiary responsible for the development and purchase of medical products. In addition, the company has sold manufacturing and distribution rights of its first device (AQUEDUCT-100) in China to a Chinese distribution company, and continues to maintain commercial relationships with them for the subsequent product (AQUEDUCT-200).

Investments in the Company by Israel's Leading Technology and Innovation Entities
Aqueduct Medical began its operations within the NGT technology incubator. After two years in the incubator, the company received additional funding from the chief scientist and the incubator as part of their “Third year” program. At the end of that year, the company received additional financial funding from the chief scientist and the incubator as part of their “Emerging Company” program.

A Team of Medical Device Development Experts, with Experience in Setting Up, Managing and Selling startups
The company has an experienced management team and a supportive medical team, led by Prof. Michael Stark – President of NESA (New European Surgery Academy). The company's CEO, Amnon Weichselbaum, served as technology and business manager of the Ofek medical incubator. He also founded and established startups in the field of medical devices. Two of the startup companies are already successfully promoting and selling their products on the market.
Pitch
Pitch
The Need
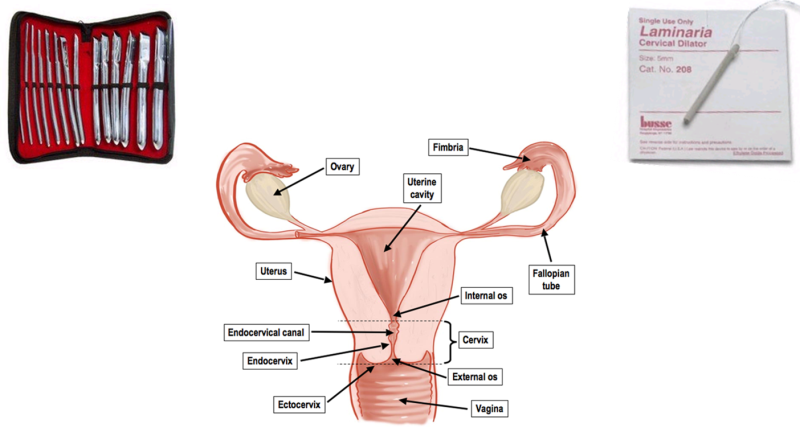
Every intrauterine procedure begins with dilation of the cervix for the purpose of inserting the surgical tools. Currently, there are two main methods of dilation:
- Mechanical dilation - Hegar Rods: Cervical dilation is usually performed mechanically by inserting and retrieving, one by one, plastic or metal rods of increasing diameter (0.2 to 1.2 cm). The rods assist the natural tendency of muscle to expand, until the desired opening is reached. This method increases the risk of cervical/uterine perforation, infection, and future cervical failure, which could cause miscarriages or premature births. The advantage of this method is that it achieves dilation within minutes.
- Hygroscopic methods – Laminaria: A second method is to insert hygroscopic material that expands by absorbing body fluids and dilates the cervix radially. This method takes a long time (minimum 6-12 hours), thereby increasing the risk of infection, and sometimes ends in incomplete cervical dilation. This method is less practical and virtually unused.
In light of the above, a real need has been identified for a new device, one that will allow radial, rapid and controlled cervical dilation to the diameter needed for gynecological procedures.
The Solution
Aqueduct Medical’s innovative, patented medical device is the only one in the world that combines the advantages of Hegar’s rods and Laminaria, i.e. radial dilation within minutes. It is based on using high-pressure balloons to inject fluid into a catheter that is first inserted into the cervical canal while the canal orifices are sealed and dilated. This enables doctors to perform a quick and safe cervical dilation without damaging the cervices or exposing the patient to infections, while at the same time, reducing the need for general anesthesia, and thereby moving the procedure from the OR to a clinic.
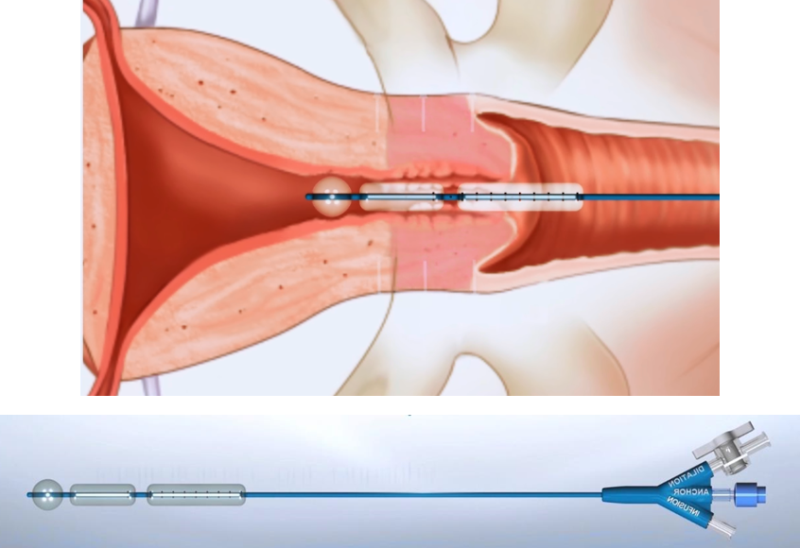
The essence of the innovation lies with the company's second device, AQUEDUCT-200, which combines the technology of the first device with a hysteroscope (a catheter with a camera), enabling doctors to both diagnose the patient and perform the dilation of the cervix and surgical treatment in the same insertion procedure.

If, after the diagnosis, it is determined that no surgical intervention is required, the physician can remove the device easily and without unnecessary cervical dilation. However, if there is a pathological finding (such as a polyp), the physician can dilate the cervix by inflating the balloon on the catheter and perform the medical procedure immediately.
This is in contrast to existing devices, which require the physician to dilate the cervix without prior diagnosis, or perform two separate procedures: the first - inserting a thin hysteroscope for diagnosis, and the second - performing cervical dilation and surgery.
The Technology
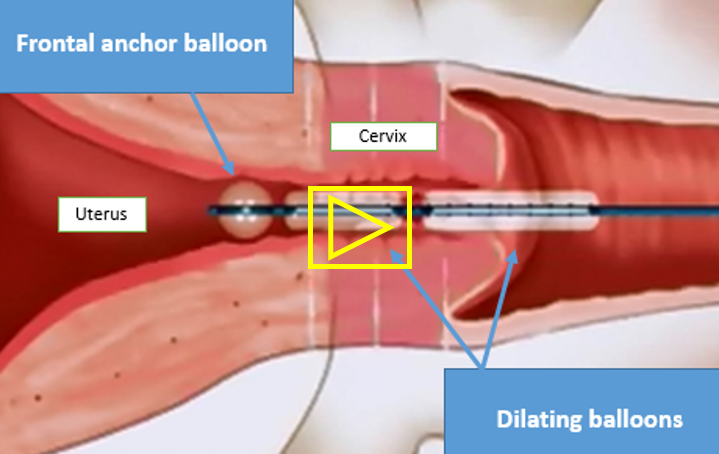
- The company's first product - AQUEDUCT-100 - is a thin, flexible catheter (3 mm in diameter) that allows easy and convenient penetration of the cervix’s natural opening. The catheter contains high-pressure balloons that allow catheter fixation and anchoring in the uterine orifice. Then the balloons, located in the cervix, are inflated through controlled injection of liquid, so that the cervix opens radially (from the center outward) to the desired diameter, allowing the gynecologist easy access to the uterus once the catheter is removed.
The catheter at the beginning of the procedure (frontal anchoring balloon and cervical dilating balloon are deflated ):

Another mechanism in the catheter is an infusion pore located between the two expanding balloons that allows fluid injection for cervical flushing and lubrication, as well as for insertion of anesthetics.

The illustration below is the catheter during the dilation procedure. The frontal balloon anchors the catheter in the uterine cavity and the two elongated balloons fully dilate the cervix.
Testimonials by doctors who participated in the clinical trials

- The company's second product, AQUEDUCT-200, is a hysteroscope (catheter with a front camera) that also includes the cervical dilatation device for use as needed. The device allows a simple and safe insertion of the catheter from the cervix into the uterus, while taking real-time images. In the case of intrauterine findings, the doctor can immediately perform the cervical dilation and begin the surgical procedure. On the other hand, if nothing is found, the doctor can remove the catheter and prevent an unnecessary cervical dilation.

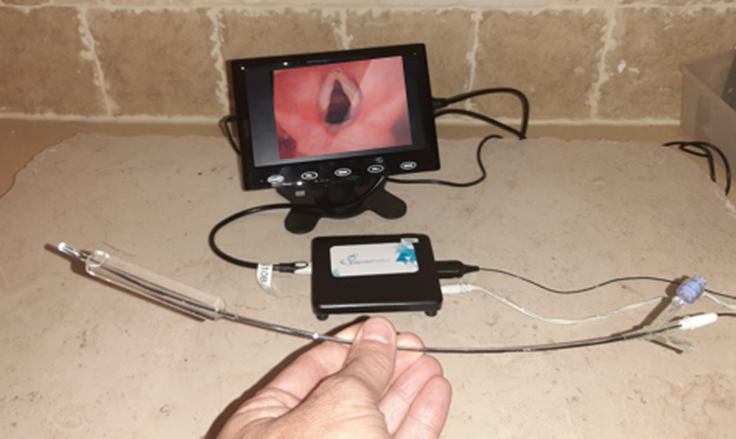
The combined device is the first and only one that can perform the diagnostic procedure (looking at the cervix and uterus) and cervical dilation according to findings with a single catheter insertion.
A significant advantage is in cases where, during the diagnostic examination, no findings are discovered requiring surgical intervention. Accordingly, the catheter is removed, avoiding unnecessary cervical dilation, which exposes the woman to complications and infections.
Articles and Publications

Team
Team
|
earned a Ph.D. in Medical Sciences, Technion – the Israel Institute of Technology and has undergone postdoctoral research at the Faulkner Center for Reproductive Medicine, an affiliate of Harvard Medical School in Boston, U.S. Amnon is a seasoned entrepreneur in the medical field. Founder of:
• Fertiligent – slow release insemination device
• Bacterioscan – electro-optical device for bacterial detection in urine
• Aqueduct Medical – Innovative cervical dilator
Amnon also served as the CTO and Scientific director at the "Ofek” Technological Incubator in Migdal HaEmek, Israel. He has over 20 years of experience in promoting and managing many medical projects as part of the CTO‘s role in the technological incubator. He has registered as an inventor 7 patents on medical devices and methods and have published over 20 articles in scientific journals.
|
|
Omer has extensive experience in managing companies in the manufacturing and medical field. He has provided consulting services, and has been involved in the management team of a number of high-tech companies. These include Palgal – plastics company for the communication market, and Tamuz – producer of electro-mechanical
|
|
Zohar is the Managing Partner & CEO of the NGT technological incubator, and Chairman of the Board of Aqueduct Medical. He has gained a wide range of experience in identifying new technologies and in establishing, directing, and creating value for technology-based start-up companies. He has led establishments and investments in more than 50 companies.
|
|
Nizar is a certified CPA, and holds an MBA from Tel Aviv University. He is the Managing Partner and CEO of NGT3, and manages the finances of all NGT3 companies. He has strong experience in handling many different complex investment transactions
|
|
Dr. Rofe, a graduate of Technion University, has been the chief executive officer of the Bnai Zion Medical Center* since the beginning of the 21st century. A physician specializing in obstetrics and gynecology, Dr. Rofe holds a degree in medical management as well. He has also served as a consultant for the organization and establishment of specialized medical projects in Israel, Britain and Africa
|
|
Dr Tzabari is a senior Gynecologist with more than 30 Years of experience. He has held the position of Head of the Department of obstetrics and gynecology for more than 16 years at the Yoseftal Medical Center in Eilat. Dr. Tzabari presents research projects in the field of Gynecology and infertility and sexual functioning medical conferences around the world. He is a member of NESA – New European Surgery Academy.
|
|
Prof. Dr. Michael Stark is specialized in Obstetrics and Gynecology. His main interest is gynecological oncology. He is the President of the New European Surgical Academy (NESA), an international inter-disciplinary surgical academy. Prof. Stark was the scientific advisor of the European novel Tele-surgical system, scientific and medical advisor of the ELSAN, a 120-hospital group in France and is a guest scientist at the Charite’s University hospital in Berlin. In 2011, Prof. Stark was nominated as the Medico Del Anno (Doctor of the Year) in Italy, and is an Honorary Member of the French, Polish, Russian and Italian Gynecological Associations. In the years 1983-2000 he was the director of Ob/Gyn department of the Misgav Ladach Hospital in Jerusalem, and between 2001 and 2009 the chairman of Gynecology at the HELIOS, European Hospital Group. He was visiting Professor at the Universities of Toronto, Moscow, Beijing, Milan, Adana, Uppsala and New York.
|