Guide In Medical
147% of funding target
Highlights
Highlights
Guide In developed a lifesaving medical device
Guide In developed the IRRIS, an advanced non-invasive medical device designed to help medical and rescue teams perform tracheal intubation (artificial respiration procedure) accurately and safely. The IRRIS enables the medical personal, whether in the hospital or in the field, to easily and safely identify the trachea of the patient, thus avoiding possible complications resulting from errors in orientation in the oral cavity.
Proven technology validated by clinical trials
The IRRIS is a breakthrough and innovative medical device with protected by patent, which is based on cooperation between the Hebrew University and Hadassah Medical Center in Jerusalem. The product was tested in four different clinical trials in Europe (Spain, Switzerland, Denmark, and Romania) with a great success in all four.
A huge market – over 80 million procedures performed each year
Each year over 80 million intubation procedures are performed worldwide. In the United States alone, this is the third most common medical procedure, with about 20 million procedures performed annually, 25 million procedures are performed annually in China. The estimated size of the US market alone is about $ 650 million annually.
Guide In has paved the way towards the market
Guide In has received the FDA's approval for marketing its product in the US, the company's main target market, and is authorized to market its products in Europe with the CE mark. The company also has received marketing approvals of the Israeli Ministry of Health and the Canadian Ministry of Health.

A leading team which has identified the need by their own experience
Guide In was established as a result of a clear need that came from the field. Each if the team members encountered this need from a different angle: an intensive care director at Hadassah Hospital, an airborne medic graduated from Unit 669, and world-renowned medical physicians. The team was joined by senior engineers and experienced business development professionals in the field of medical devices.
Proven success – Support by The Israeli Innovation Authority and of a leading pharmaceutical company
Guide In is a graduate company of the Technological Incubator and VC (NGT3) of the Israeli Innovation Authority and has also received additional development grants. Additionally Guide In has signed a strategic partnership agreement with the CTS group, one of the most prominent companies in Israel engaged in the healthcare, agricultural and veterinary sectors, in which CTS invested in the company. Guide In is currently in negotiation with a well-known international company, one of the world's leading companies in the fields of emergency medicine, anesthesia and intensive care by an agreement of exclusive international distribution of the device.

Pitch
Pitch
The Need
Tracheal intubation is a common medical procedure (the third most common medical procedure in the United States) where a breathing tube is inserted into the patient's trachea to supply artificial ventilation by mechanical or manual respirators. This procedure is performed daily in patients in the operating room before surgeries, as part of general anesthesia and as a life-saving procedure in treatment of wounded and patients in emergency rooms, intensive care units and trauma departments in hospitals and emergency medicine (ambulances and military settings).
In this procedure, the challenge of the physician, paramedic or nurse is to quickly identify the tracheal opening, separate it from the esophagus opening (leads to the stomach), and insert a breathing tube fast and safe into the trachea (the breathing tube is than connected to a respirator). Today, optical devices with a video camera (called video-laryngoscopes / fiber optic video) are inserted into the patients' throat to assist in locating and visualizing the trachea and inserting the breathing tube.
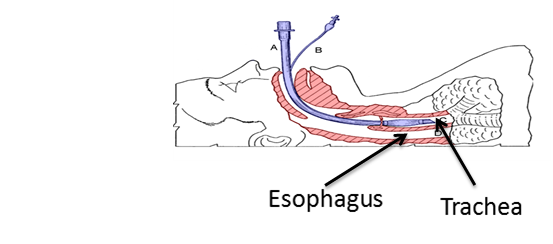
It is crucial to identify the trachea and insert the breathing tube as fast as possible since the patient doesn't breath at the time of the procedure. Delay in the insertion of the breathing tube or misplacing it into the esophagus (instead of the trachea) may cause serious injuries and medical complications due to lack of oxygen and even death in extreme cases. There are many factors that affect the difficulty in performing the procedure, trachea identification and breathing tube insertion such as:
• Patient's anatomy (e.g. Small or unusually large jaw, short neck, limited mouth opening)
• Risk populations such as obese patients, children, pregnant women, oncology patients, ear and throat surgeries , maxillary surgeries.
• Skill and experience of the physician / paramedic / nurse
• Work environment (operating room VS emergency room or field medicine)
• Trauma cases (restricted head movement and mouth opening which are necessary for tracheal identification)
• Blood and gastric secretions which worsen visualization and identification of patient's the trachea
In cases where there are difficulties in identifying the trachea, the insertion of the breathing tube may take time. In more severe cases, the breathing tube may accidently misplaced into the esophagus and cause complications such as lesions of the soft tissue of the throat, dental injuries, hypoxia, brain damage and even death. Tens of millions procedures in hospitals and in the field are performed worldwide annually; the IDF found that the success rate of the procedure is only 39%. Studies found that in the emergency room there are complications in 25% of the cases, in intensive care units and rescue & emergency services there are complications in 40% of cases.
ILTV Interview
תוכנית רק בריאות בערוץ 12
The solution
Guide In Medical was established by the intensive care director at Hadassah Mount Scopus Hospital in Jerusalem and an airborne medic graduated from Unit 669 (combat airborne rescue & evacuation unit of the IDF); based on their personal experience in the hospital and in the field. The motivation for establishing the company was finding a simple, inexpensive, non-invasive solution that would simplify the procedure, reduce the chances of medical complications and save lives. The company, which has a medical ISO quality standard, is currently developing a number of products relating to the various stages of respiration of patients and wounded in hospitals and in the field, in collaboration with the world's top key opinion leaders in this field. The company's IRRIS device was developed on the basis of technology that was obtained under exclusive license from the Hebrew University and the Hadassah Hospital in Jerusalem.
Links:
Geek Time
http://www.geektime.co.il/guide-in-medical-raised-2millionils/
The Times of Israel
http://www.timesofisrael.com/israeli-medical-startup-raises-nis-2m-for-life-saving-trachea-device/
The Pulse
https://thepulse.co.il/36815-2017-01-26-07-14-10
לינק לראיון ב-:Tech Time
https://techtime.co.il/2018/02/05/guide-in-medical/
בדה מרקר:
http://www.themarker.com/technation/1.3432880
http://www.il-israel.org/nl/170205/index.html (בגרמנית)
https://nepmag.com/izrailskie-mediki-pridumali-pribor-oblegchayushhij-intubatsiyu/ (ברוסית)
https://www.diariodecadiz.es/provincia/luz-final-garganta_0_1089491557.html (ספרדית)
The Technology
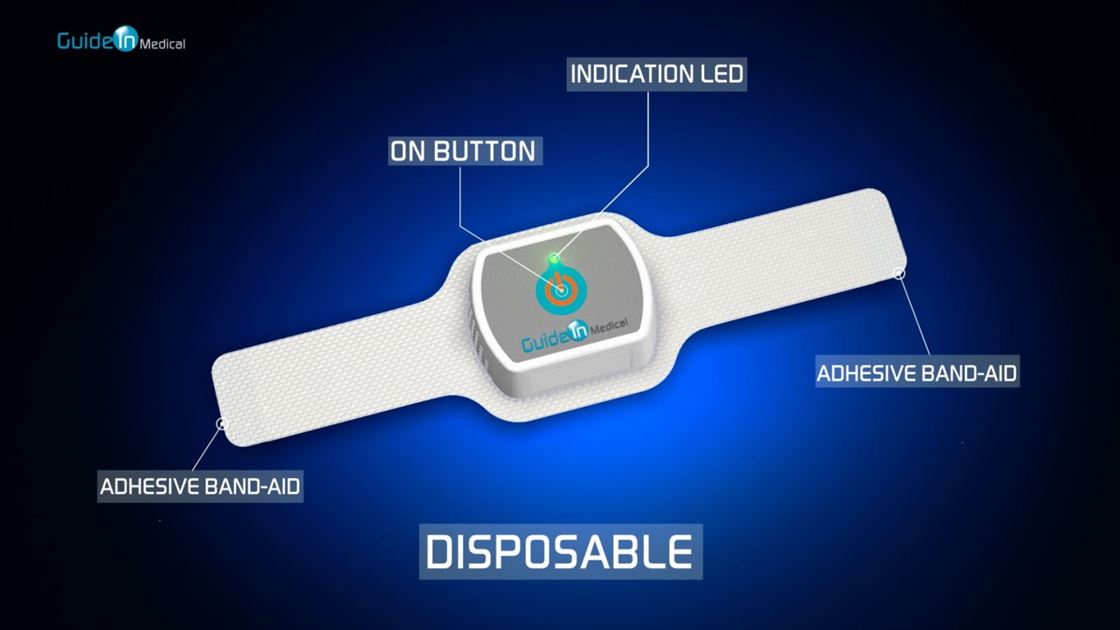
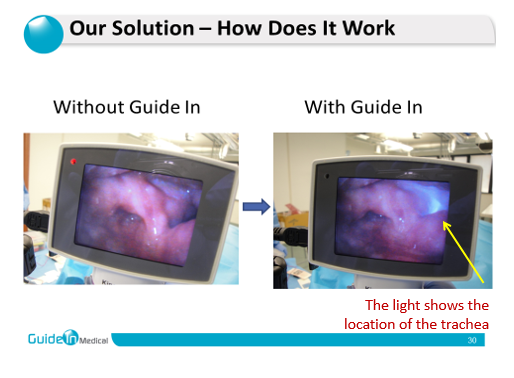
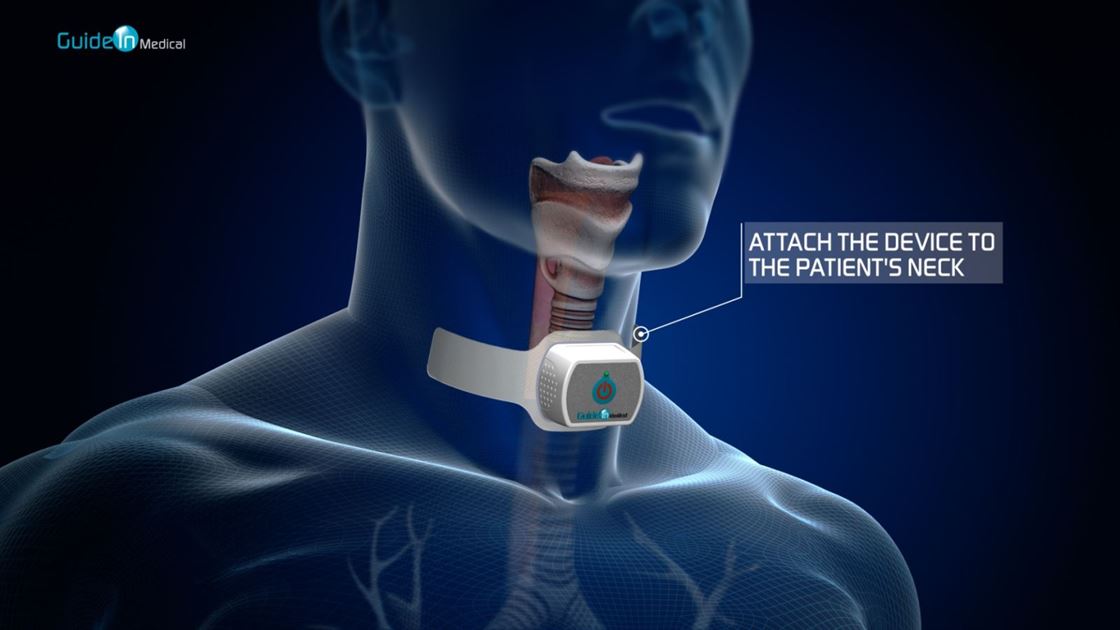
Guide In has developed a unique, non-invasive medical device-the IRRIS patent protected, which is a new approach to tracheal intubation procedure. The product was developed based on technology licensed from the Hebrew University and Hadassah Hospital in Jerusalem, enabling the physician, paramedic or nurse to identify the trachea of ??the patient and casualties quickly, accurately and safely, thereby reducing potential complications. Guide In's guided intubation device is based on a noninvasive electronic illumination patch, which is placed on the patient’s neck. The device transmits infra-red (IR) light to the underlying tissues. The light penetrates the neck tissues safely and emitted back solely by the trachea while it is absorbed by the surrounding tissues, thus enabling the clear identification the trachea position even in difficult situations. In this manner the trachea is marked, illuminated and clearly marked and distinguished from the esophagus which located beneath the trachea, therefore reduces the chances of misplacement of the breathing tube. Nowadays, physician use optical devices with a video camera (called a video-laryngoscope and fiber optical video) which are inserted into the patient's mouth to identify and visualize the trachea and insert the breathing tube (these devices already exist in medical institutions). When using the innovative IRRIS device, the IR light emitted from the patient's trachea is identified by the video device and is clearly seen its video-screen illuminating specifically the trachea. All the physician has to do is follow the light (indicating the location of the tracheal opening) and insert the breathing tube into the trachea. The light actually gives the physicians guidance to the correct place.
demonstration video
pictures from clinical trial
לינקים מניסויים קליניים
https://drive.google.com/file/d/0B4vkr6N8NJJaLXFaQkFjT2p4U0k/view?usp=sharing
The IRRIS is patent protected and is clinically proven in a number of clinical trials conducted with world-leading KOLs. The IRRIS has FDA, CE, and Israeli Ministry of Health and Canadian Ministry of Health marketing approvals. The light emits and flashes from the trachea helps locate it and differentiate it from the esophagus even in complicated situations where visualization is impaired (due to blood or anatomical difficulties for instance).
Advantages:
• IRRIS is intuitive and easy to use (all it takes is to apply the device upon the patient's or casualty throat and press the power button) and suitable for both hospitals and emergency medicine in field conditions.
• Immediate identification of the trachea through the intubation process
• Non-invasive device which is completely safe and dose not endanger the patient, the device can only improve the clinical situation and benefit both the therapist and the patient.
• Paramedics and military physicians who are not sufficiently experienced in the procedure are those who are required to provide medical aid to casualties in the field under time pressure. The IRRIS allows untrained and experienced medical personals in the intubation procedure to quickly locate the patient's trachea and provide lifesaving mechanical ventilation.
• IRRIS also helps in complicated and difficult situations in hospitals.
Team
Team
|
Ariel has vast experience in emergency medicine as a graduate of the Israeli Air Force (IAF) elite airborne rescue and evacuation unit (Unit 669). He brings managerial experience and project management skills in the medical-device and pharmaceutical industries.
Ariel is a graduate of the prestige Merage Institute Leadership program for life science & medical devices.
Ariel holds a B.Sc. in Biology and an MBA (with distinction) specializing in marketing management and strategy & entrepreneurship, both degrees from the Hebrew University, Jerusalem.
|
|
Nizar has hands-on financial management experience at all levels: from seed investment through early and late financial rounds. Managing partner and CFO of NGT3vc early stage fund. Nizar is a certified CPA and holds an MBA from Tel Aviv University’s Recanati program.
|
|
Dr. Fried is the head of the ICU in Hadassah medical center, Mt. Scopus. He had Residency and fellowship in Hadassah‘s medical centers, and critical care fellowship in Toronto, Canada. He was an instructor and physician in the IDF medical corps. Elchanan has great Knowledge and experience in emergency medicine.
|
|
Israel Lax: has served in numerous executive sales positions in Johnson & Johnson, ETview and MST.
Mr. Lax‘s professional experience includes sales & marketing management, Business development and product launch of medical devices internationally.
Mr. Lax holds a master‘s in law from Bar-Ilan University and a BA in economics and business management from Ben-Gurion University of the Negev.
|
|
Nir Tsouri has over 15 years of experience in medical device and military avionics companies. Nir has extensive experience in Engineering, Manufacturing, Logistics and Purchasing, QA, ERP systems and customer service. He has worked in operations and customer support and was USA Subsidiary Director of Operations in medical device companies. Nir served as VP Operations at Ornim Medical (specializes in the development and distribution of medical devices in the field of noninvasive cerebral blood-flow monitoring). He was VP Operations at ITGI Medical (symbol ITMD in TASE) – the company that brought to the world heterologous tissue covered stents for Cardiovascular and Neurovascular interventions. Nir was the Director of US Operations at Itamar Medical (symbol ITMR in TASE), which engages in the development and sale of non-invasive medical devices for the diagnosis of cardiological conditions and sleep breathing disorders.
Prior to that, Nir was with Elbit’s Systems Operations division as technical trainer, specializing in military avionics.
Nir holds a B.Sc in Electrical and Electronic Engineering of the University of Hertfordshire, Hatfield, UK.
|
|
Director of the anesthesia and intensive care unit at Carmel Hospital and vice president of the European anesthesiology society.
In his managerial roles, Dr. Goldik served as Deputy Director of the Carmel Hospital until 1995. In addition, he serves as chairman of the hospital‘s doctors‘ committee, a position he has been full of dedication and loyalty for three consecutive terms. Dr. Goldik brings great honor to the State of Israel and the Carmel Hospital in his roles throughout the world of international medicine. Dr. Goldik is the vice president of the European anesthesiologist society. He has been the European anesthesia test chair for 10 years. He is also president of the European Council for Medical Examinations, an international advisory body based in Brussels and a
consultant to the EU.
|
|
Peter Biro is a senior consultant and a senior lecture at the Institute of Anesthesiology, University Hospital Zurich and leads the division of Anesthesia in Urology. He has focused on problems connected to airway management, thus presenting profound knowledge, experience and skill in the management of the difficult airway and prevention of hypoxemia, brain damage and anesthesia related mortality. Besides he was involved in developing the standards of acute post-anesthesia care, obstetric anesthesia and pharmacological questions. The habilitation in 2001 focused on clinical aspects of High Frequency Jet Ventilation. Peter Biro has published over 40 original articles, 25 review articles, 14 book chapters and 7 monographs. The number of invited lectures in various national and international institutions is exceeding 250. Peter Biro has received the Teaching Recognition Award of the European Academy of Anaesthesiology 2001. yoel.
|
|
Director of the Anesthesia Advanced Airway Management Fellowship at Toronto General Hospital, Canada.
Dr. Cooper’s primary professional interest relates to advancing airway management devices and techniques as well as enhancing clinical documentation. In this regard, he was actively involved in the development of a video laryngoscope (GlideScope) and two commercial airway exchange catheters (Cook Airway Exchange Catheter and CardioMed EndoTracheal Ventilation Catheter, ETVC®). He was a founding member of the Society for Airway Management (SAM) and served as its president from 2012-2014. Dr. Cooper also served as chair of the program committee and on the organizing committee of for SAM 2012 and the World Airway Management Meeting 2015 respectively. He is currently the director of the Anesthesia Advanced Airway Management Fellowship at Toronto General Hospital. He was co-editor of The Difficult Airway—An Atlas for Clinical Management (Springer, 2013)and has also been actively involved in the development of the Canadian Airway Focus Group Guidelines (1998 and 2013) in addition to consulting for the development of the ASA and Difficult Airway Society airway management guidelines. Dr. Cooper had published numerous book chapters, original articles, and editorials and has been an invited speaker throughout North, South and Central America, Europe, Asia and Australia. He has most recently been a refresher course lecturer at the International Anesthesia Research Society and the ASA Annual Scientific Meetings (2015).
|
|
Former CEO and director of Beta-O2 Technologies for a period of 8 years, brought the company to one of the world leaders positioning in the area. Served as CEO of the Technion Entrepreneurial Incubator (TEIC) for a period of 11 years. Led the establishment and investment in more than 40 companies, such as Prolor Biotech (sold to Opko, NYSE: OPK, for $480M on August 2014), Mazor Robotics (NASDAQ: MZOR, TASE: MZOR.TA, www.mazorrobotics.com), ReWalk (NASDAQ: RWLK, www.rewalk.com), Corindus (www.corindus.com), Regentis (www.regentis.co.il), BEE (www.beei.com) and many others. Zohar Gained a wide range of experience in identifying new technologies and in establishing, directing, and creating value for technology-based start-up companies.
Zohar Served as a member of the Technion’s Patent Committee (1998 – 2006).Earned his M.Sc. in material engineering, a B.Sc. in physics, and a certificate in business management, all from the Technion.
|
|
Over 20 years of managerial experience in the medical device and healthcare industries. Dr. Ben-Josef is the former CEO of ETView Medical, an airway management medical device company that he took public in 2010 (TLV: ETVW) and led to 3 consecutive public offerings. Former Medical Director at Itamar Medical Inc. (Framingham, MA), US subsidiary of an Israeli publicly traded medical device company (TLV: ITMR). Former VP Corporate Affairs and IP at X-Technologies Inc., a US medical device company acquired by Guidant Corp. in 2003.
|
|
Managing director of Jacobs Investment Company LLC, San Diego, California. Proven track record of over 10 years of investment in life science start-ups and technological incubators. He serves as chairman of the board of DermTech International, Nutrinia Ltd., GEO2 Technologies Inc., and is on the board of Fallbrook Technologies. He is the Chairman of the Board of Trustees of High Tech High (HTH) Graduate School of Education and the National High School Reform movement
|
|
Ido Helft joined CTS in 2010 as CFO. In his previous position, he served as CEO at Tiv Taam company.
|
|
Itai has broad experience in medical R&D. He is a PHD candidate in electro-optics in the Hebrew University of Jerusalem. One of his significant achievements includes the development of a sensor needle. Itai graduated Singularity University summer program at NASA Ames research center in the Silicon Valley and he‘s the co-founder and CEO of Scopio labs a company in the field of microscopes.
|
|
Leon holds a bachelor‘s degree in electronics and computer engineering. Leon is responsible for the electronic and software development in Guide In‘s products, and is a partner in the design of the production line.
|