
Iluria
A technological revolution in ADHD treatment management
115% of funding target
Highlights
Highlights
Making ADHD Treatment Smart and Accurate
Approximately 10% of children and adolescents worldwide are diagnosed with ADHD. However, medication effectiveness and safety are subjectively measured through trial and error and retrospective questionnaires. This outdated approach often results in unsuitable treatment, wastes time and resources, and ultimately lowers engagement. Iluria's technology is a breakthrough for children, parents, and caregivers, by providing continuous and objective analysis of medication treatment processes through mathematical analysis of physiological indicators (e.g., heart rate, movement, etc.), measured by commercial smartwatches. This patent-protected technology is the first of its kind in the field.

Transforming a Billion-Dollar Market: Innovative Solutions for Tremendous Medical Cost Savings
Currently, over 250 million people worldwide are diagnosed with ADHD, with approximately half of them being children and adolescents aged 5-19. In the US alone, the cost of treating this condition can reach tens of thousands of dollars per patient annually. Unfortunately, much of this cost is wasted on incorrect and ineffective treatment processes, resulting in a significant financial burden for healthcare providers, insurance companies, employers, and families. Additionally, some of these costs are not covered by insurance, which poses difficulties for the families of patients and leads to high rates of abandonment of drug treatment. Iluria's revolutionary technology offers a scientific approach to accurately treating ADHD, preventing financial losses, and improving outcomes for patients and their families.

Clinical Trials and Global Pilots with Leading Medical Centers
Iluria's technology has already been through successful clinical trials with Clalit, Israel's largest HMO, and the Children's Hospital in Cincinnati, a world leader in the field of ADHD. The company has begun pilots with several notable medical centers across the world. These include:
- Rotherham Doncaster and South Humber NHS Foundation Trust in England
- Cincinnati Children's Hospital, USA
- IRCCS MEDEA Associazione La Nostra Famiglia in Italy
- Bethesda Children's Hospital in Hungary
- Tel Aviv University

In addition, Talks are underway with other centers such as the Central Institute of Mental Health in Germany and the University Medical Center Groningen in the Netherlands. The company is also exploring a strategic collaboration in the English market with Takeda, one of the largest pharmaceutical companies globally. These collaborations aim to evaluate the effects of drug treatment accurately and ultimately reduce medical costs while improving patient outcomes.
Patented Technology
Iluria's technology is protected by an international patent (PCT/IB2020/050088) already approved in the United States and under review in Europe and other markets. Additionally, the company has submitted a patent application (PCT/IB2022/052516) to extend the use of their technology to depression and anxiety. Iluria is working on filing another patent in the field of mental health to further invest in their breakthrough technology. These patents solidify Iluria's position as a leading innovator in the mental health space, with the potential to offer meaningful solutions to a wide range of mental health conditions.
Streamlined regulatory procedures
Building upon successful clinical trials and pilots, Iluria is advancing through the FDA's DeNovo pathway for approving its technology as a decision support tool for ADHD diagnosis. Furthermore, collaborating with Rotherham Doncaster and South Humber NHS Foundation Trust in the English market will involve seeking UK regulatory approval for the solution. Concurrently, the company is also preparing to launch a non-medical product version in 2024, catering to private schools and individual users in the United States.
Accelerators, Competitions, and Prizes
Iluria's dedication to driving global innovation in the mental health space has been recognized through its participation in international accelerators and contests, resulting in the company winning several prizes
.


Prominent Investors and Grants
Iluria is backed by prominent global investors and investment organizations. Moreover, the company secured a grant from the Israel Innovations Authority and a financial award from EIT Health (backed by the European Union).

Pitch
Pitch
In the recent past, children and adolescents with ADHD faced the burden of stigma, being referred to as "having ants in their pants." They were often considered poor students, and sometimes even labeled as "problematic." Today, the health and education systems recognize that attention deficit disorder (ADD and ADHD) is a genuine and chronic neurodevelopmental condition resulting from dopamine deficiency, and is unrelated to a student's intelligence or cognitive abilities.
The attention disorder manifests in short-term symptoms of inattention, hyperactivity, and impulsivity. Improperly treated attention disorders, particularly during the early stages, can lead to long-term negative consequences, including:

Essentially, the very systems meant to aid these children often contribute to a "self-fulfilling prophecy" through unfair labeling.
Presently, approximately 128 million individuals aged 5 to 19 worldwide are diagnosed with attention deficit disorder, accounting for nearly half of the total diagnosed population of around 250 million people.
Behavioral treatment alone is insufficient for most ADHD cases and typically necessitates regular medication to balance dopamine levels. There is a wide variety of drug treatments, with different release mechanisms, doses, and more. Analyzing ADHD is challenging for the following reasons:
• Treatment begins with physicians prescribing the lowest available dose of a chosen drug, which may be adjusted or changed over several months or even years.
• The effectiveness of drug treatment varies individually and evolves alongside physiological changes in patients.
• The disorder encompasses distinct aspects - inattention, hyperactivity, and impulsivity - which respond differently to drug treatment. For instance, while hyperactivity levels may decrease with medication, the child's attention may not improve significantly.
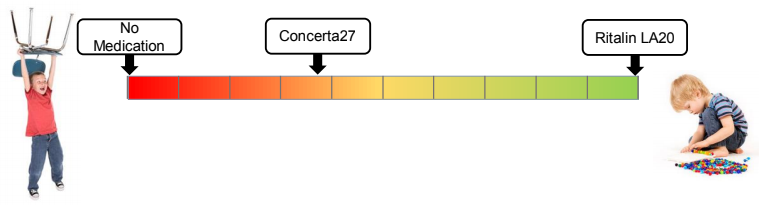
Current monitoring processes rely on manual questionnaires. These subjective methods involve input from teachers and parents, who may lack the relevant skills to assess treatment efficacy. As a result, the monitoring process remains trial and error-based, leading to suboptimal clinical outcomes, low engagement rates (less than 50% within the first two years), and substantial costs borne by clinical service providers and insurance companies. Annual treatment expenses for a child with ADHD in the US reach tens of thousands of dollars, similar to the costs level in Europe. Furthermore, parents spend additional non-covered costs, exceeding one thousand dollars per patient.
The Solution
Iluria's software introduces a revolutionary technological solution that offers a constant and objective evaluation of drug treatment safety and efficacy. This is achieved through mathematical analysis of physiological indicators recorded by any wearable IoT device or smartwatch owned by users.

Using machine learning-based algorithms, the system assesses the physiological impact of drug treatment throughout the day, including duration and intensity, while also providing long-term trends. The monitoring process is entirely passive and non-invasive, requiring no active participation from the patient.
The goal of Iluria's software platform is to enhance treatment quality, foster patient commitment and adherence, and reduce ongoing treatment expenses. It achieves this by delivering objective and continuous monitoring in accordance with the recognized clinical standard, the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5).
On June 1, 2023, the company secured a patent for their ADHD solution in the American market. Similar processes are underway for other regions such as Europe, China, India, and more.
How it Works
Iluria's software utilizes mathematical analysis of daily fluctuations in physiological markers, which have already been scientifically and medically established to be associated with ADHD. These markers undergo changes during the course of drug treatment processes, including movement, angular movement, heart rate, heart rate variability, and more. Physiological data is routinely collected using various intelligent wearable devices such as Fitbit or Apple Watch. The initial product version employed Samsung Galaxy watches for pilot programs conducted in medical institutions, with plans to incorporate additional smartwatch and wristband platforms.
The mathematical analysis of physiological data is performed by the machine learning engine, which delivers a continuous evaluation of the therapeutic impact throughout the day. This includes identifying risk factors like abnormal heart rate increases and assessing long-term treatment trends. Objective reports generated through passive patient monitoring are provided to relevant stakeholders, including clinicians, patients, and parents.

The core component of the system, the machine learning technology, underwent testing in two completed clinical trials conducted by the company. The first trial was successfully conducted in collaboration with Clalit Health Services, while the second study, involving two measurement stages, partnered with the Cincinnati Children's Hospital. Cincinnati Children's is one of the leading pediatric medical facilities in the United States and a world leader in ADHD research and treatment. Additionally, the company initiated another clinical trial in Italy in March 2023, collaborating with IRCCS Eugenio Medea - Associazione La Nostra Famiglia, a leading Italian children's hospital. Furthermore, the company is initiating additional field trials (pilots) in the United States, England, and Europe.
Selected from a pool of companies, Iluria secured a partnership with Takeda Pharmaceutical, through the Meet the Pharma Startups program organized by EIT Health. Additionally, the company had the opportunity to present its solution to Takeda UK's management, exploring potential collaboration in the British market.
Looking ahead, the company is actively working on expanding the solution to address ADHD in adults as part of its product strategy. In that regard, the company is starting a pilot with Tel Aviv University to measure treatment impact on the student population. Moreover, in line with the future product roadmap aimed at treating other behavioral disorders such as anxiety and depression, the company recently submitted a patent application covering the management and control of drug treatments in these areas.
Technology and intellectual property
The company's technology being developed for attention deficit hyperactivity disorder (ADHD) is unparalleled worldwide, providing continuous and unbiased monitoring through existing technological infrastructures, such as smartwatches, without the need for external intervention.
In collaboration with Clalit Health Services, the company conducted an experiment to develop the initial version of the product. This version enables the identification of treatment-related situations based on clinical diagnosis and medical factors. To avoid biasing of misdiagnosed cases, a group of patients underwent three DSM-V compliant clinical diagnostic processes, with and without drug treatment. Simultaneously, the mathematical models' different layers were developed to automatically assess the effectiveness of drug treatment solely through physiological measurements using smartwatches. The results of this successful clinical trial were presented at the annual conference of the Israeli Society for Clinical Pediatrics (for more information, click here).
In the United States, a successful clinical trial was conducted with Cincinnati Children's Hospital. This trial involved the expansion of the technology into a multi-day analysis of drug treatment tailored to each individual patient.
On March 2023 the company launched another clinical trial with the Italian base IRCCS Eugenio Medea - Associazione La Nostra Famiglia, with the goal to provide additional data to enhance the models.
In January 2019, the company submitted a PCT (global protection) application to safeguard intellectual property in the ADHD field. The application is currently in the National Phase (for more details, click here). On June 1, 2023, the US Patent Office confirmed the approval of the patent for the USA. Additional territories are expected to grant final approvals in the upcoming year.
Furthermore, in March 2021, the company submitted a PCT application to protect intellectual property in the domains of anxiety and depression. These areas also involve a trial and error process in monitoring the effectiveness of drug treatment (for more details, click here).
International collaborations
Institutional medical service providers
Alongside the collaboration with Clalit Health Services, the company has partnered with Cincinnati Children's Hospital, renowned as the foremost institution in the United States specializing in attention disorders. This collaboration includes a multi-year plan for product development and commercialization, integrating with the organization's core system for managing treatment processes in the field of attention disorders,the Cincinnati Children's Hospital Mehealth for ADHD center (www.mehealth.com).
In the UK, the company collaborates with a medical center affiliated with the English Health Authority (NHS) - Rotherham Doncaster and South Humber NHS Foundation Trust. The collaboration involves various stages, including a product pilot and support for regulatory (CE-UK) and commercial (UK Reimbursement) processes. Given the relatively favorable regulatory environment in England, the company is considering an initial launch of the medical product in the U.K. market before expanding to the U.S. market.
Pilots and additional clinical trials: As mentioned, the company initiated a field pilot in partnership with a prominent medical center in Budapest, Hungary - Bethesda Children's Hospital. Concurrently, an expanded clinical trial is being conducted with one of Italy's leading medical centers, IRCCS Eugenio Medea - Associazione La Nostra Famiglia.
Simultaneously, the company formed a consortium for clinical and commercial collaboration with several leading medical centers in Europe, including the aforementioned centers in Italy and Hungary, as well as the Central Institute of Mental Health (Germany) and University Medical Center Groningen (Netherlands). The consortium aims to jointly pursue grants from the European Union and carry out additional pilots.
To establish the future infrastructure for analyzing the effects of drug treatment in adult patients, the company initiated a clinical trial with Tel Aviv University. This trial focuses on analyzing the treatment's effectiveness for young adult (students), with the primary objective of preventing unnecessary overuse of various drugs as a means to enhance concentration.
Technological platforms for parents of children with ADHD
While evaluating direct-to-consumer activities, the company has established collaborations with various companies, including:
- A partnership with Tiimo (www.tiimoapp.com), a Copenhagen-based company specializing in developing technologies for the daily management of ADHD. Tiimo's product presently caters to over 50,000 end users.
- Collaboration with Neuro-solutions (www.neurosolutionsgroup.com), a Canadian company that created a video game targeting behavior regulation in ADHD patients. The company serves approximately 50,000 end users.
- Iluria serves as a partner in the consortium led by Amazon Web Services. This consortium aims to create a comprehensive system, named ADHDMyWay, to fully support parents of children with ADHD in managing their unique challenges. Within this framework, Iluria's role focuses on incorporating the scientific and clinical components into the system.
Pharma companies
- The company was chosen to participate in the Meet the Pharma program organized by the EIT Health organization, paving the way for potential collaboration with Takeda, a global pharmaceutical company. Currently, we are exploring opportunities to join forces with Takeda UK in our endeavors within the English market.
- Furthermore, the company maintains ongoing communication with other pharmaceutical companies, including Otsuka, to explore potential partnerships and collaborations.
Iluria in the media
- Mckinsey's - People Beyond Healthcare Innovation
- Selected as one of the winning startups in the Innovate Children's Health Challenge II organized by the Center for Advancing Innovation;
- Iluria was selected as one of the finalists at the Children's National's second annual Bear Institute Pediatric Accelerator Challenge for Kids (PACK) innovation challenge, Children's National is one of the leading pediatric hospitals in US;
- Selected by Takeda as a finalist in the 'Takeda UK ADHD Innovation Challenge Accelerating personalized ADHD healthcare';
- Iluria has been selected to present to the 2nd E-Health Investment Forum;
- Reached the 2nd place in the 11th ite ration of LvlUp Ventures pitch perfect competition.
Team
Team
|
Hagay has been a founding partner and CEO of Iluria since it was formed. Hagay has over 25 years of business c-level experience in various high tech companies in the B2B world, in the fields such as Fintech, Communications and Homeland Security. Hagay has held a wide range of management positions, such as CFO, Business Development Manager and Product Management. At the beginning of his professional career, Hagay gained extensive experience as a business analyst, specializing in corporate recovery. Hagay has a BA in economics and management and a master‘s degree in finance and accounting, both from Tel Aviv University.
|
|
Ran has extensive experience in accompanying, maturing and leading technology companies at an early stage, mainly in the field of medical equipment and life sciences. Prior to joining Iluria Ran served for many years as an investment manager at an investment firm in the venture capital sector, during which time he accompanied companies such as Lithotech Medical (now owned by Nordson Medical) MediGuide Ltd. (now owned by Abbott) and Modelity Technologies. Ran has a BA in law (LL .B) from the Interdisciplinary Center in Herzliya.
|
|
Birkat is a founding partner of Hoshen Project Management, one of Israel‘s leading companies in the country in the fields of urban renewal, urban planning, infrastructure and structures.
Birkat, who serves as a director at Iluria, founded the company together with her life-partner, Hagay Levy, based on many years of in-depth knowledge in the field of ADHD, as a mother of 4 children, 3 of whom are diagnosed with attention disorders.
Birkat has a BA in landscape architecture from the Technion and a MA in business administration from Tel Aviv University.
|
|
Prof. Liran Carmel is a world-renowned mathematician and scientist in the field of computational biology. Liran is the director of the Department of Genome Research at the Hebrew University and completed his postdoctoral research in the US at the National Health Institute, specializing in molecular evolution, genetics and RNA biology. In addition to his academic background, Liran has many years of experience in developing technologies for various industries. Liran accompanies Iluria since prior to its formal establishment and helps formulate and test the scientific and mathematical concept on which the technology is based. Liran has a BA degree in physics from Tel Aviv University, a MA in physics from the Technion, a doctorate in mathematics and computer science from the Weizmann Institute of Science, and a postdoctoral fellowship from the National Health Institution in the United States.
|
|
Prof. Itai Berger is an expert in pediatrics, pediatric neurology and child development, and is one of the leading experts in the country in the field of attention disorders. Itai currently manages the child neurology services in the pediatric ward at Assuta Ashdod Hospital. Itai studied medicine at Ben Gurion University, specialized in pediatrics at Shaare Zedek, then specialized in child neurology, at the Children‘s National Medical Center in Washington, D.C. and at Shaare Zedek Hospital. He also managed the child neurology unit at Hadassah Hospital.
|
|
Noam is an expert in data science and leads the development field in the company. Noam is a graduate of 8200 and holds a Ph.D. in machine learning and anomaly detection, with extensive experience in data mining, big data learning, and algorithmic development based on machine learning.
|





