
Implant-b
13% of funding target
Highlights
Highlights
The Only Definite and Proven Solution for Peri-Implantitis
The ImplantB dental implant solves the problem of PI in dental implants. PI is a common
inflammatory disease that affects up to 40% of dental implants each year, causing great
suffering to the patients and, in many cases, leading to physical removal of the implant.
The company has successfully completed a pre-clinical trial in Spain - the first in the world to
show bone regenration around the implant (re-osteointegration), and is currently starting
its first clinical trial.
Innovative, patented dental technology
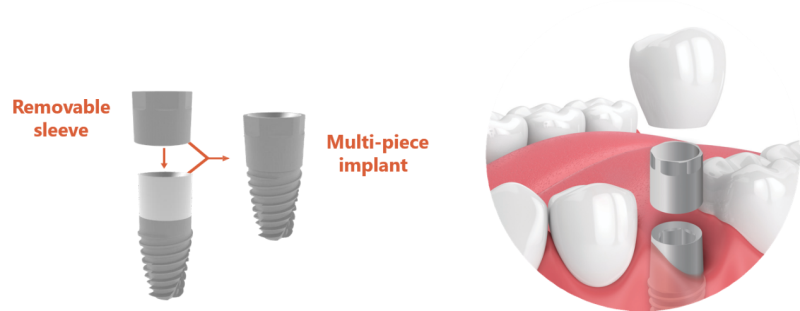
Implant B developed the NGI 100 - an innovative dental implant made up of two parts - a body and a removable sleeve, which allows the easy replacement of contaminated surfaces. The implant can be suitable for use by all dental implant companies, and does not directly compete with existing implants. NGI 100 provides a unique solution for periemplantitis, which does not currently exist in the dental implant market. The company has approved patents in the US and Europe.
An Unmatched Product in a Huge and Extremely Profitable Market
The dental implant market is estimated at billions of dollars a year and growing rapidly.
Implant B has developed a unique implant that gives a unique solution to a problem that has
not yet been solved in the implant market. Implant B's product is ready for mass production,
its production costs are not high and the market is very profitable, with tens of millions of
dental transplants performed every year.
Implant B is in the process of getting regulatory approvals from the FDA and a European standard certificate from the CE.
.png)
Support of a Prestigious Technology Incubator and the Israel Innovations Authority
Implant B developed its technology within the NGT3 Technological Incubator, and has,
therefore, also received funding from the Israel Innovation Authority, which supports and
invests in early stage medical technology companies. The company initially received a
significant multi-year grant for the development of its technology, and it received an
additional grant later on.

A Team of World-Renowned Experts in the Field of Dental Implants and Start-Up
Management
Implant B was established by top dentists who are closely acquainted with the problem of
peri-implantitis in dental implants and who identified the market need for an effective
solution. The company's staff consists of senior dentists, engineers, and management and
investment professionals with extensive experience in establishing, managing and investing
in start-ups.
Pitch
Pitch
The Need
Peri-implantitis is an inflammatory disease that occurs in approximately 10% - 36% of dental implants each year (in high-risk patients, such as smokers, diabetics, chronic patients, etc., the incidence can reach 45%).
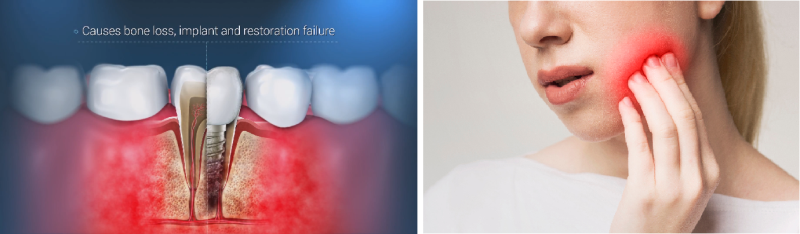
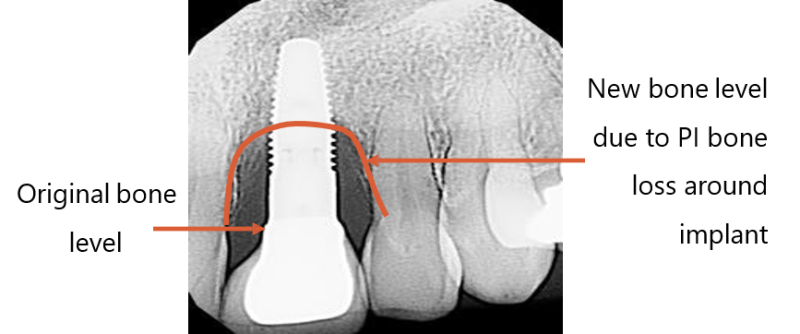
The disease starts in the gums’ soft tissue, moves to the bone and causes bone loss, implant and restoration failure. The implant is exposed and infected, making it necessary to remove it and begin the whole process anew, all the while knowing that the disease may very likely recur.
Current solutions try unsuccessfully to focus on implant surface decontamination and physical removal of pathogens. Today, there is no solution in the market that solves the problem of PI and prevents the need for re-implantation.
The Solution
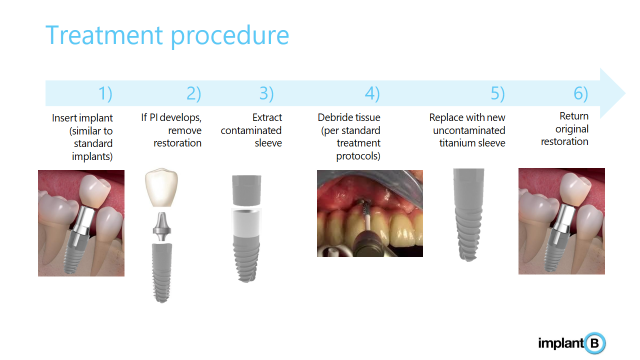
Implant B's innovative implant is made up of a Titanium body and a removable sleeve. The sleeve sits on the upper part of the implant and thus prevents the disease from affecting the implant. In other words, instead of the bacteria infecting the implant itself, it infects the sleeve. The sleeve can be easily replaced as soon as an infection is detected, thus preventing the need for re-implantation.
In the event of PI, all that is required is to replace the sleeve with a new clean sleeve. This solution maintains the original location of the implant as well as the original reconstruction (tooth or bridge).

The Technology
The dental implant consists of two parts made of titanium - a body and a removable upper sleeve that protects the implant against bacterial accumulation. In the case of PI, the infected sleeve is removed, the infected area is cleaned, and a new, clean sleeve is placed.
This implant is protected by a patent approved in the US and Europe and is the only one in the world that provides a solution for Peri-Implantitis.
Team
Team
|
Hagai has 15 years of experience in management, business development, sales and marketing management in leading companies in the business sector.
For the past 6 years, he has been managing Medigma Biomedical GmbH, a German company that manufacturing and marketing dental implants. Hagai managed Medigma operations, which included managing two subsidiaries and managing operations in 20 countries around the world.
Prior to that he served as a business development consultant for companies that wanted to open activities in the world in the fields of agriculture, food, plastics and medical devices.
Hagai holds a BA in Political Science and Communication from the University of Haifa and a Master‘s degree in Management and Strategy from Tel Aviv University.
Hagai also serves as a director of "Zohar Dalia" and brings with him extensive business experience in setting up companies and setting up operations around the world, and in-depth knowledge of the market
World Dental Implants.
|
|
Specialist in Periodontics. Graduate of the internship program at Tel Aviv University.
Graduated with honors from Tel Aviv University.
MA in Medical Sciences.
She has published more than 15 studies in the professional literature. Her main areas of research include: soft tissue healing processes, soft tissue around implants, treatment of perimenaplasty and rheumatoid augmentation.
Liat also conducts clinical and biologic research at Tel Aviv University.
|
|
B.Sc Mechanical Engineering and M.Sc. in Biomedical Engineering from the Technion, M.Sc. Quality Assurance from Haifa University.
Ludan company - Mechanical Engineer in Process Engineering.
Iscar company - Quality Assurance Manager at CNC Plant.
Mis company -Project Manager in Operations and Engineering.
Adir Gadiokov is an expert in the fields of product development, transfer from development to production and quality assurance.
|
|
Managing Partner & CEO of NGT 3 which is an early-stage investor that operates technological incubator and invest in medical technologies. NGT? portfolio currently includes a diverse portfolio of 18 companies.
Former CEO and director of Beta-O2 Technologies (bio-artificial pancreas).
Zohar served as CEO of the Technion Entrepreneurial Incubator and Led the establishment and investment in more than 50 companies, such as Prolor Biotech (sold to OPKO, for $480M), Mazor Robotics (sold to Medtronics,for $1.6B), ReWalk (NASDAQ: RWLK), Corindus (NASDAQ: CVRS) etc.
|
|
Director, Oral and Maxillofacial Surgery Department, Rabin Medical Center, Petah Tikva, Israel.
Director of Oral and Maxillofacial Surgery at Beilinson Hospital.
He is a professor in the Department of Surgery at the School of Dental Medicine at Tel Aviv University where he served as the department‘s director.
He managed the Oral and Maxillofacial Unit at Ichilov Hospital and Assaf Harofeh Hospital.
Member of the Scientific Council. Served as secretary and chairman of the Society of Surgery, Member of the Professional Committee of Surgery, Head of Oral and Maxillofacial Surgery.
Prof. Gabi Chausho teaches many courses in Israel and abroad and has published over 150 articles; In addition, Forbes Magazine has been selected Gabi for several consecutive years as one of the best mouth and jaw specialists in Israel.
|
|
Chairman of Dept. of Periodontology & Implantology, Tel-Aviv University.
The Gerald Niznick Chair of Implant Research. Former Dean of the University School of Dental Medicine.
Honorary member of the American Academy of Oral Medicine
Visiting Professor at the Universities of Toronto, Pennsylvania and New York.
|
|
Chief of Oral and Maxillofacial department, Galilee Medical Center., Nahariya, israel.
|
|
|






