Lydus Medical
314% of funding target
Highlights
Highlights
A Breakthrough Medical Device, Protected by Three Registered Patents
Lydus-Medical has filed three patent applications (in advanced stage patent pending): The first protects the general concept, while the other two protects the unique needle insertion mechanism developed by the company and the customization of miniature diameters. The patent portfolio is diverse, broad and covers the entire developed platform.
Investments in the Company by the Leading Innovation, Medical and Med-Tech Entities in Israel
Lydus-Medical was founded by Mor Research Applications, the technology transfer office of Clalit Health Services, the largest HMO in Israel. Immediately at the company's founding, it received a significant investment from Sanara Ventures and the Innovation Authority's incubator program. Sanara Ventures is a leading medical incubator in Israel and an investment arm of Philips Healthcare and Teva. The company has also received an investment from AMIT, a private fund of the Technion, which invests in the development and commercialization of exceptional biomedical technologies.

Quick Advancement from Idea to Product Stage to Beginning of Commercial Activity
Lydus-Medical has successfully developed a working surgical device within a period of only two years, demonstrated its safety and efficacy in successful preclinical trials, and is generating considerable commercial interest. The company has a LOI (letter of intent) from a US distributor that specializes in microsurgery and directs a wide network of agents throughout the US, with understandings of sales volume and final product price for the first 3 years.
Enthusiastic Responses from the International Medical Community
In recent weeks, Lydus-Medical attended the annual ASRM (American Society of Reconstructive Surgery) convention at Miami, USA, where the device was tested under surgery-simulating conditions by several leading physicians and received an exceptionally enthusiastic response regarding the accuracy of the coupling.

Simultaneously, Lydus is in advanced discussions for strategic collaboration with NIHR - the National Institute for Health Research in England. The institute is investing major resources to bring new medical technologies into the country. The collaboration objectives is a funded clinical trial of the Lydus device at Leeds Hospital, which will speed up regulatory processes in Europe.
The Technology as a Platform - Implementation in a Wide range of possible devices
The company's technology will potentially serve as the basis for a variety of devices suitable for various medical applications, and which will have uses in various types of surgery. In the first stage, the company's symmetrical needle-insertion mechanism is implemented for open-access surgery (reconstructive surgery, organ transplantation, peripheral bypass, and others). Subsequently, it can also be applied to robotic and laparoscopic surgeries (penetration of the abdomen through a number of small abdominal incisions, surgically supervised by a camera).
Pitch
Pitch
Every year, some 250 million surgeries are performed worldwide. In order to improve surgical results, the medical world is increasingly adopting ever more innovative solutions for the automation of surgical procedures. However, despite being the most complicated part of the surgery, anastomosis, which is coupling of ultra-small blood and lymph vessels and the key to the success of many of these procedures, is still done manually. When Performed manually, these procedures are time and labor intense and requires high surgeon dexterity and unique skills set, often result in poor clinical outcomes.
The Need
Anastomosis , coupling of ultra-small blood and lymph vessels are delicate and complex procedures, which can take many hours of strenuous work in an operating room and have a steep learning curve. Stitching small blood vessels of 0.5-4.0 mm diameter is performed under a microscope and must be extremely accurate in order to achieve good clinical results. Perfectly symmetrical coupling is critical to the success of many surgical procedures since it ensures proper blood flow.
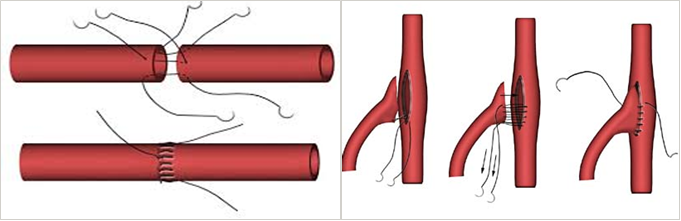
The clinical success of coupling depends on many parameters, but mainly on the symmetry between the needle points on the blood vessels diameter, and a uniform symmetry between the needle point and the tip of the blood vessels. Lack of symmetry can cause leakage, which is the most common risk, but not the most serious, as it is detectable and repairable. A more serious problem of asymmetry is turbulent flow formation in the coupling area, which requires further surgery and, in rare cases, can even lead to death.
Today the procedure is performed manually , making it time and labor intensive, requiring high surgeon dexterity and a very unique skill set. This reality often results in poor clinical outcomes
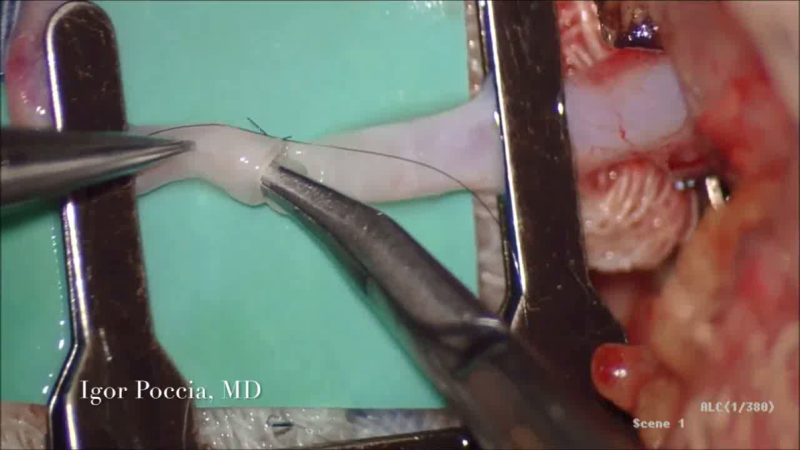
The Solution
Lydos Medical has developed the VeSeal, an innovative surgical device that enables the automatic, symmetrical and perfect stitching of 0.4 - 0.5 mm vessels in complicated procedures such as breast reconstruction, organ transplants and bypass surgery.

Technology Advantages:
1. Safe – delivering accurate and standardized anastomosis
1. Intuitive, easy and fast.
2. Saves time in the operating room.
3. Reduces the need for highly superior surgical skills.
4. Can be used for all kinds of hollow organs: arteries, tiny blood vessels and lymphatic vessels.
5. High safety profile - Reduces the tendency of blood vessels to warp and twist and prevents ischemia, blockages and leaks.
6. Leaves no foreign body
6. Eliminates the danger of accidental back wall stitching .
7. Can be performed by a single doctor (as opposed to 2 in manual operations)
8. Does not require a microscope in the operating room – microscopes can be replaced by a magnifying glass (microscopes are expensive and are not available in every operating room).
9. The only available solution on the market today for coupling of tiny vessels (0.5-0.4 mm), one that will allow these procedures to be performed in many medical centers that currently lack the qualified staff.
The idea is to develop utilities that will allow any surgeon without specific microsurgery training, to perform safe anastomosis of tiny blood vessels.

The invention is based on the existing manual method of surgical sewing, using thread and needle, which is safe and effective but eliminates the technical difficulty involved. As mentioned, the most important parameter for safe coupling is the symmetry between the threads. This is also the most complicated part to perform, as it involves placing 150-micron miniature needles under a microscope, around a 2-mm-diameter blood vessel.
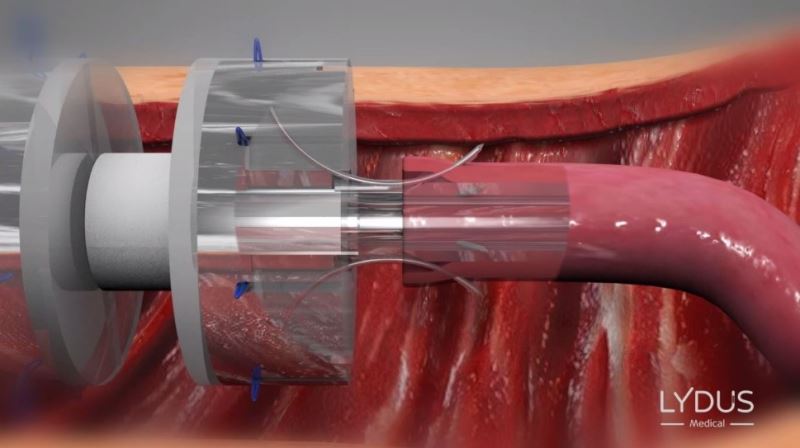
The technology developed by Lydus Medical meets these requirements. The disposable surgical tool, which comes pre-loaded with multiple microsutures and needles, keeps the blood vessels dilated at all times while the needles are being inserted (thus avoiding the common risk of back wall stitching), and symmetrically and simultaneously places the needles and threads in the optimal place. The coupling results when using the device are identical to perfect manual performance.
The Technology
The technological innovation is in the needle-insertion mechanism, which is based on a combination of unique bending at the tip of the needle and a lever-based principle, as opposed to other, propulsion-based mechanisms. From a functional perspective, the innovation is in eliminating the technical difficulties of the manual suturing, which allows for shortening and improving the process, as well as making coupling possible for any surgeon to perform.
Steps of Procedure:
Positioning the device between the two edges
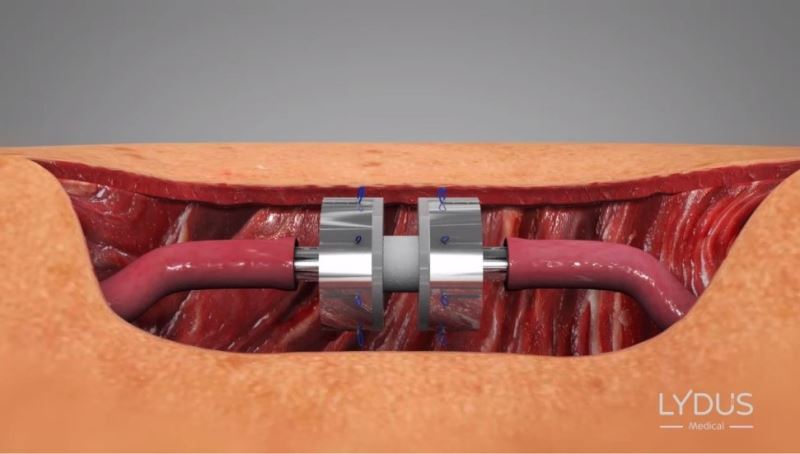
Symmetrically and simultaneously inserting the 8 needles around the periphery of the blood vessels
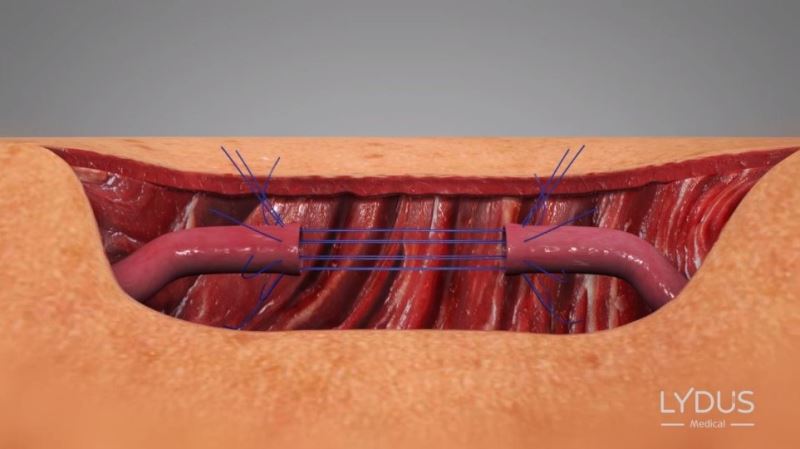
Pulling the needles out
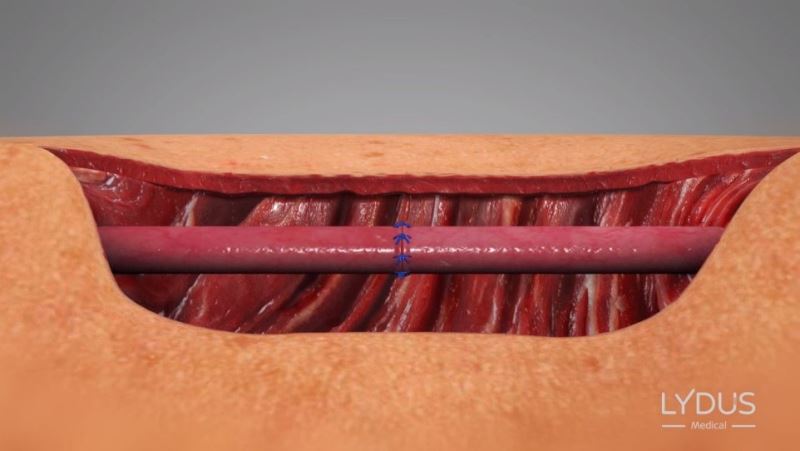
Knots are performed in the traditional method
Team
Team
|
Biography
Head of department of plastic surgery Rabin Medical Center Studies: Graduate of the Technion School of Medicine with honors (1988) Location: Hadassah University Hospital, Jerusalem Courses: Sloan Kettering Hospital, New York (1996-7) Academic appointment: Senior Lecturer - Tel Aviv University Lecturer - Hebrew University Visiting Professor - Yangzhou, China Memberships in unions and professional organizations: Israeli Plastic Surgery Association American Plastic Surgery Association (ASPS) European Plastic Surgery Association (EURAPS) International Aesthetic Surgery Association (ISAPS) The Israeli Society for Microsurgery (Chairman, 2009-2002) Israeli Society of Surgery and Oncology Head Neck Special areas of interest: Breast reconstruction Microsurgery and neck head reconstructions Malignant skin tumors Aesthetic surgery advanced technologies Previous clinical experience: Director of Micro Surgery, Neck Head Reconstruction and Cleft Surgery in the Department of Plastic Surgery, Hadassah, Jerusalem. Research activity: Skin tumors, wound healing, mathematical algorithms for disease processes, advanced aesthetic medicine technologies.
|
|
Biography
Developer and inventor in numerous fields, owns over 100 patents. An experienced engineer with extensive experience in the medical device industry, from concept to product. Mr. Kamall has extensive experience in developing products from various disciplines and has over 100 approved patents. He is also very active outside of Israel, in Europe and the US. Kamall‘s Experience and connections are reflected in the development process in terms of engineering knowledge of design, international connections for production and required testing.
|
|
Biography
Specializes in plastic surgery and burns at Beilinson Hospital. An entrepreneur and active partner in the design and execution of preclinical trials.
|
|
Biography
Mechanical Engineer with 8 years of experience in medical devices. |






