
Veoli
50% of funding target
Highlights
Highlights
Breakthrough Technology with Two Registered Patents
The unique technology developed by VEOLI uses mechanical energy to turn cannabis extraction-based liquid into a spray (aerosol). It delivers a precise dose without heating, evaporation or burning. The spray production process (nebulization) is based on a company-developed combination of a unique oil-free formulation and an innovative electronic inhaler. Veoli currently hold two registered patents in Europe USA and Israel; a third patent for the cannabis formulation and capsule will be filed within the coming time.
An Innovative Medical Development, Supported and Financed by the Israel Innovation Authority, Sanara Ventures and Merhavia Holdings
The technology was originally developed for the pharmaceutical field in the Sanara Ventures technology incubator and funded by the Israel Innovation Authority .Shortly following its founding in early 2019, Veoli received its initial significant investment from current major share holders, Sanara Ventures (the investment platform of Teva and Philips Healthcare), which is also one of the company's founders and its largest owner was the first investor. This investment was promptly followed by another noteworthy investment, by Merchavia Holdings and Investments, an Israeli public company which concentrates on early-stage biomedical and life sciences companies.

An Experienced Team in the Fields of Medical Device, Pharma, Oncology and Pain Therapy, with a History of IPOs and Exits
Veoli is led by a multidisciplinary team of entrepreneurs and advisers with decades of experience in the fields of medicine, medical device development, pharma, business development and management, and with an impressive record of establishing companies and bringing them to commercialization, acquisitions and IPOs. CEO Tzur Di-Cori was part of a small team who established Card Guard Scientific Survival, which later after several years launched an IPO on the Swiss stock exchange, and later renamed LifeWatch AG. Kobi Ben-Efraim served as the company's CFO for 10 years. Tzur Di-Cori was also one of the founders of G-Medical, which launched an IPO after only two years on the Australian Stock Exchange. The CEO has established / managed other successful companies including EZSurgical Ltd, Step Of Mind, Motorika Ltd and more.

The Technology as a Platform
Veoli's technology will serve as a platform for the world's leading cannabis extraction companies. Those companies will be targeted as Veoli’s potential business partners and will use their own liquid-based cannabis products as the basis of Veoli's unique formulation, to be consumed as automatically released aerosol, using Veoli's electrical cannabis inhaler device. Veoli will offer its partners to modify their existing liquids based cannabis products to oil-free products consumed by inhalation. Veoli’s unique formulation is suitable for each type of cannabis extraction and medical cannabis use. Thus, Veoli does not compete with manufacturers; it offers them a new and advanced application for any of their existing cannabis products/brands.
A Rapidly Growing Market with Unlimited Potential
The medical cannabis market is one of the most significant market, and expected to grow significantly in the years to come. The high growth rate can be attributed to the wide range of uses of medical cannabis, as well as increasing legalization worldwide.
Pitch
Pitch
Cannabis has been used for medicinal purposes for thousands of years and in many cultures around the world. Its many therapeutic properties include relieving symptoms of chronic pain, nausea, loss of appetite and PTSD, and even, some claim, curing illness.
In recent years, cannabis has become the preferred solution for many patients who are reluctant to use prescription narcotics, which are addictive, in many cases less effective, and often have serious side effects.
The growing use of cannabis for medical purposes, along with regulatory changes in many countries, including Canada and the United States, is expected to make the cannabis market one of the most profitable in the world in less than a decade.

Need and Solution
Medical cannabis can be consumed in several ways: smoking, inhalation, eating, drops and pills. However, each of these methods has significant drawbacks, among them - toxin formation, inaccuracy of dosage, slow and ineffective absorption, sore throat, bitter taste that discourages use and more.
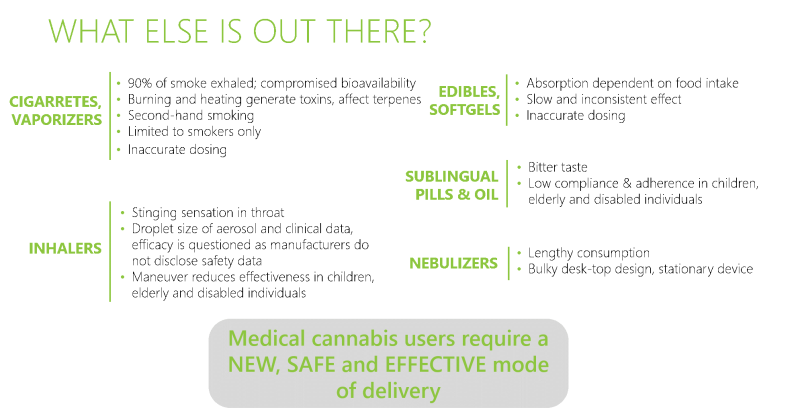
If cannabis could be inhaled effectively and measurably, without smoking and without evaporation and it's accompanied toxins, many patients would receive effective treatment while retaining only natural substances; neither the patients nor their environment would be harmed.
In recent years, a number of technologies have been developed that seek to promote and refine inhaled medical cannabis (evaporation, smoking), but all of them are deficient in the transmission of the substance, the dosage and the accuracy of the treatment. Veoli's technology solves the variety of problems involved in effectively transferring the active substance to the lungs:
1. The products available in the market use burning, heating or evaporation, all of which damage the natural substance (cannabis) and eliminate much of its medical effect.
Veoli's technology does not involve burning (like a cigarette), heating or evaporation of the active substance and therefore does not harm the natural ingredients. Veoli's product produces an aerosol employing only mechanical energy, and using a unique formulation developed by the company.
2. The process of combustion and / or evaporation creates toxins, leaving both the patients and the people around them exposed.
The dose inhaled with Violi's device is accurate because it is not smoke or vapor. It is a fluid that turns into spray and is absorbed effectively through the lungs into the blood, as applied in the pharmaceutical world. Thus, the amount of active substance inhaled is absorbed entirely, as opposed to evaporation or smoking, where most of the smoke entering the lungs is not absorbed. Since the spray(aerosol) is produced by using only mechanical energy, without burning or evaporation, no toxins and by-products are produced in the process.
3. With today's existing solutions, medical efficacy is low, as only a small percent of the substance is absorbed into the blood and most of the smoke is exhaled. The dosage is inaccurate, uncontrolled and does not allow for personalized treatment. There is no control over the rate of smoking and absorption of the active substances into the blood.
With Veoli's technology, the activity of the device is automatically synchronized with the inhaling activity of the patient. Inhaling aerosol via lungs has the most effective bioavailability. The inhaled aerosol is fully absorbed into the blood, thus enabling "economical" and effective use of active ingredients.
In summary - With smoking or evaporation, the smoke or vapors created by heating the cannabis are inhaled and exhaled, so that very little is absorbed by the lungs, in an unmeasurable and uncontrolled manner. In addition, not only does the combustion of the cannabis damage and alter the ingredients, but it also introduces toxins into the inhaled material, which is harmful to the patients and their environment.
Custom Treatment - The dosage of the active ingredient is provided in a precise and customized capsule. Veoli’s capsules include an RFID chip which contain all data about the manufacturing date dosage and concentration of the cannabis ingredients as prescribe personally to the user, usage timetable and puff control etc. Since the substance in the entire capsule is absorbed into the blood seconds after inhalation, dose accuracy is maximally controlled and is specific for each patient.
Veoli's device will enable two-way Bluetooth communication; the product will report to a medical service center via smartphone, allowing full control of patient care and compliance while including the patient in the process.
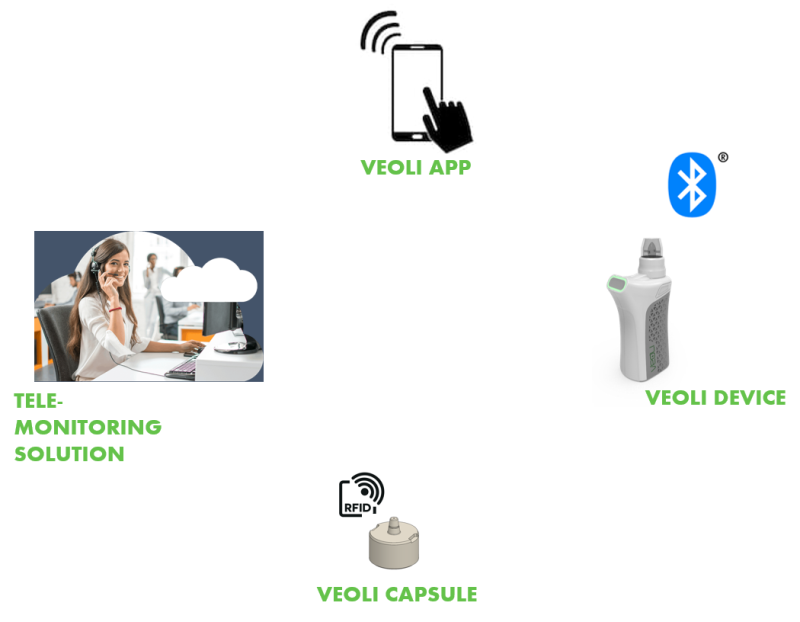
The Technology

Veoli's technological innovation comprises two parts:
1. A unique oil-free liquid formula developed by the company, based on cannabis oil -free extract infused with other medicinal substances, stored in a dedicated capsule.
2. Electrical cannabis nebulizer: Mechanical energy-based technology, which turns the liquid into inhalable spray (aerosol) without burning, heating or evaporation. The inhaler is loaded with a unique cartridge containing capsules holding an exact amount of formulation fluid. Each capsule has an RFID chip ID number which allows the device to read its exact data, such as: date of manufacture, expiry date, ingredients, dosage, suitability for customer, and so on.
The technology is an innovative technology for providing medical cannabis inhalation spray.
Using mechanical energy, the device enables the decomposition of the cannabis liquid-based formulation and transforms it into tiny droplets of 0.001 - 0.005 millimeters in the form of aerosol (a gentle spray).
The process of changing cannabis-based liquid to aerosol does not involve heating, evaporation, or burning the cannabis, allowing for the preservation of active ingredients and maximum clinical effect. The tiny drops, up to 5 microns [0.005 millimeters], enter the lungs and proceed into the bloodstream, a process that produces an immediate medical effect. The device emits an accurate dose of the substance, which is absorbed entirely into the blood through the lung vapor. Smoke or evaporation, on the other hand, enters the lungs and is exhaled with almost no absorption into the blood through the lungs, thereby requiring a large amount of uncontrolled and non-filtered smoking. Veoli technology does not create smoke or steam but only clean liquid spray. Veoli is the only company today that allows the transfer of medical cannabis in the manner described above.
The product includes the inhaler and the cartridge with the capsules. Each capsule contains a unique, precise and uniform formulation of medical cannabis composition produced under strict and stringent conditions (GMP level production). The sterile liquid in each capsule adheres to and meets medical standards. The inhaler provides the patient with measured amounts of active substance, thus allowing control and accuracy of treatment. The measurable dosage consumed using the inhaler is consistent, and the dosage and daily treatment regimen are individually tailored to each patient.
Reproducibility - The formulation is composed of cannabis ingredients in line with the Ministry of Health specifications, a specific medical indication, and the approved Ministry of Health process (GMP). The composition of the active substances is measured before the formulation is made and is based on a predetermined cannabis strain; thus the contents of each capsule are reproducible.
Consuming medical cannabis in this way maximizes the therapeutic effects of the substance and relieves pain quickly. The technology eliminates exposure to smoke and vapor damage. The inhaler is comfortable to grip, simple to operate and allows for discreet use.
Status
Steps made, and achievements so far:
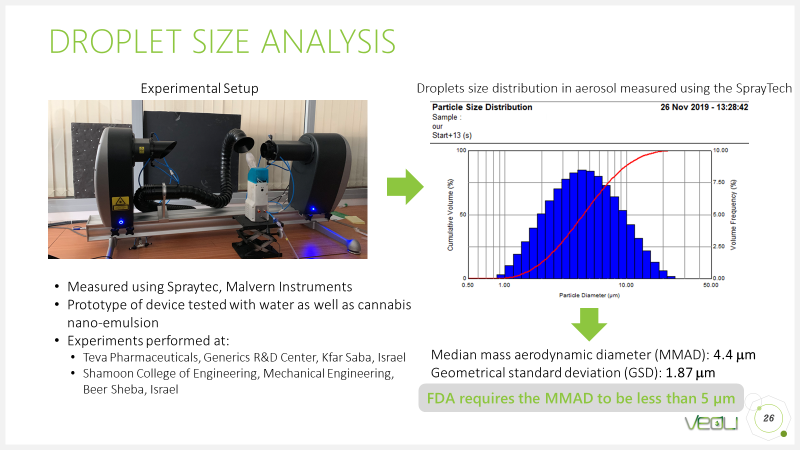
- Violi has developed a laboratory model based on the unique technology that enables the creation of a very thin spray of cannabis extraction. The aerosol (very thin spray) looks like steam or smoke produced from the liquid by applying only mechanical energy - without heating or combustion. In order to obtain aerosol droplets that comply with the strictest standard for lung aspirants, a laboratory experiment was performed on laser-based equipment (Spray Tech Lab Test Equipment) which can measure the size of the aerosol droplets. The following is an example report showing the aerosol droplet size distribution derived from laboratory sample cannabis extraction. 1 micron to 5 micron droplets were obtained as required by the FDA standard.
2) The company conducted a preclinical study in which the end-product mechanism was tested in combination with a PK study of several animals at different doses. The experiment was successfully completed and cannabis was found in the blood tests throughout the 24 hours.
3) Veoli designed the final device containing a Nebulizer (unique smart inhaler) and a capsule containing the unique Veoli formulation for daily use. Product planning of stage is completed and ready for stage ii design finetuning and transfer to production. Several working products, in final design, are being used for dose fine tuning study and testing.
4) A formula based on oil-free CBD was developed for the preclinical trial. The formulation was tested for stability and sterilized before use. The formula was developed as a platform that will enable the company to collaborate with business partners in various markets engaged in the manufacturing and distribution of medical cannabis, with the aim of converting existing cannabis-based liquid solutions/brands for different medical purposes, into inhalation solutions/ products.
Next Steps Based on Fundraising:
- Completion of design stage ii and transfer to production assembly of 50-100 final products and capsules with sterile formulation for clinical trial - final design
- The company has installed a lung simulator (Cascade Impactor NGI Lung) in the formulation development lab with the aim of completing the dose precision phase. This step will also prepare the product for submission to the highest medical standards, as required by pharmaceutical companies. The laboratory dosage and accuracy tests will be conducted using the Veoli device and the Veoli formulation.
- A second preclinical trial to validate laboratory precision testing
- Longer term formulation stability tests
- A clinical trial with a few dozen subjects and blood tests (PK study)
- Preparations for regulatory submissions in Israel, Europe and the United States in Phase B. At this stage, the regulatory strategy will be formulated.
- Transfer to Production - Production of final product molds for the device and capsule
- Develop a remote control and control management app
Team
Team
|
Seasoned executive with over 25 years of experience in the medical device space.
|
|
Senior executive with over 20 years of experience in corporate finance including private financing, IPO, follow on offerings and strategic collaborations
|
|
PhD in Medical Physics, over 25 years of experience in medical device development in both academia and industry, Talpiot graduate
|
|
Experienced executive with over 20 years of experience in life sciences including innovation, investments, business development and sales & marketing
|
|
Over 15 years of experience in the pharmaceutical industry, notably with TEVA pharmaceuticals. Shaul’s PhD thesis was focused on medical cannabis and how to emulsify it. He is currently EVP & COO at Polypid, Member of the Board Of Directors at Univo Pharmaceuticals LTD and also serves on the Advisory Board of Sanara Ventures.
|
|
Medical Director of Palliative Care at William Osler Health System in Brampton, Toronto.
Specialist in both Oncology and Palliative Care with over 25 years of experience in palliative care. Currently holds professorships at the Universities of Toronto, McMaster, Ottawa and McGill. Received the Elizabeth J Latimer Prize in Palliative Care from Mc Master University and Kay Blair Community Service Award, William Osler Health System
|
|
Director of the Chronic and Acute Pain Center, Tel Aviv Medical Center, Israel. Over 20 years of experience in pain management, licensed to administer medical cannabis in Israel, published numerous research studies on medical cannabis. Chairman of the Anaesthetists Association of Israel, member of The Israeli Association for pain treatment and IASP.
|






